췌장낭성종양의 수술적 치료대상과 수술 후 추적관찰
Surgical Indications and Postsurgical Follow-up Strategy for Pancreatic Cystic Neoplasm
Article information
Abstract
췌장의 낭성종양은 영상 검사의 보급과 발달에 따라 그 빈도가 늘어나고 있다. 이 낭성종양의 특징을 잘 이해하고 악성으로 진행할 여지가 있는지에 대한 판별은 중요하다. 내과, 영상의학과 그리고 췌장외과 간의 협력 및 논의를 통하여 종양에 대한 평가와 치료 결정이 적절하다. 특히 종양에 대한 검사 해석이 부적절 시 불필요한 수술까지 초래하기 때문에 세심한 검사와 정확한 해석이 중요하다. 췌장낭성종양의 특징과 경과에 대한 고려를 통하여 개별 환자에게 알맞은 적절한 검사 및 수술 여부 결정을 하도록 해야겠다.
Trans Abstract
The increasing discovery of pancreatic cystic neoplasm is a recent trend because of the widespread use and development of imaging techniques. Physicians have to recognize the different characteristics of the cystic neoplasms so that a determination may be selected regarding the potential for malignancy. Appropriate evaluation of pancreatic cystic lesion includes a multidisciplinary approach involving gastroenterologists with experience in endoscopic ultrasound, radiologist, and pancreatic surgeons. The selective approach is important in management of this neoplasm with minimizing incorrect diagnosis and unnecessary surgery. Considering the characteristic features of pancreatic cystic neoplasm, the clinical decision should be tailored according to needs and conditions of the individual patients.
INTRODUCTION
The main issue regarding the treatment of pancreatic cystic neoplasms (PCNs) is the inability to exactly determine the histopathologic diagnosis without surgical resection. Owing to development of imaging examination, the majority of patients who are referred to the surgeon for a pancreatic cyst do not need a precise histopathological diagnosis. Many PCNs have the various potential for malignancy as precancerous lesions. This potential was shown to be very variable, ranging from high (the risk of invasive carcinoma associated with main duct intraductal papillary mucinous neoplasms [IPMNs]) to, in some cases, extremely low (like in small, indolent branch duct IPMNs) or even almost absent (like in serous cystadenoma) [1-3]. Because of this variability, it is some challenging for clinicians to balance their practice between the risk of surgical overtreatment and the critical error of surveillance with a malignant lesion [4].
MAIN BODY
Each patient should be carefully evaluated with demographic and radiologic features. Surgery also plays a role in the critical decision, as pancreaticoduodenectomy and distal or central pancreatectomy bear a substantially different burden in terms of morbidity, mortality, and quality of life.
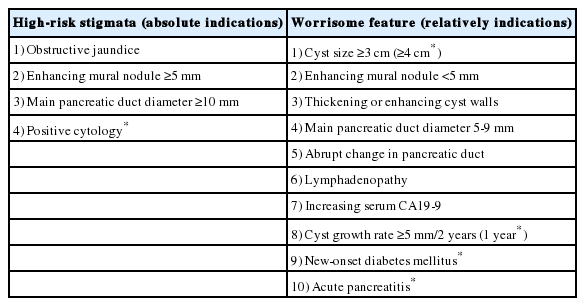
For the criteria for surgical indication in mucinous PCNs (IPMNs and MCNs), the International Association of Pancreatology 2017 and European 2018 guidelines are widely adjusted and accepted in most countries [5,6]. Guidelines are useful tools for identifying the relative risk for harboring cancer, but they must be applied with flexibility and with the awareness that, at the present time, they are mostly based on expert opinion and not fully supported by strong scientific evidence. However, the efforts of powerful evidence are still being studied. The final surgical decision should be tailored to the individual patient, considering all the variables including age, comorbidities, and risk of cancer. These guidelines are summarized in Table 1 with focusing on indication for surgery. ‘High-risk stigmata’ or ‘absolute’ indications for surgery in all fit patients include PCN-related jaundice, the presence of a vascularized mural nodule or solid component, and a cytology suggesting malignancy. Surgery is also recommended for all fit patients presenting with a main pancreatic duct (MPD) dilation >1 cm, only if there is a high suspicion of main duct or mixed IPMN (no signs of MPD obstruction or chronic pancreatitis). Other features, such as MPD between 5 and 9 mm or a progressively increasing MPD dilation, recurrent acute pancreatitis, a cyst size ≥30 mm or a rapid growth rate (>2.75 mm/year), thick vascularized cyst walls and increased serum levels of CA19-9, are all features of concern but are not strong predictors of malignancy. Patients with suspected features but without ‘absolute’ indications for surgery should undergo contrast-enhanced endoscopic ultrasound; if the malignancy suspicion is not confirmed endoscopically, patients should undergo close follow-up with magnetic resonance imaging/magnetic resonance cholangiopancreatography, oncological markers and contrast-enhanced endoscopic ultrasound when indicated. This EUS should be considered for further evaluation of mural nodules, and is also helpful in assessing vascularity within the cyst and septations [6]. The single pictures of each observation provide an appraisal of the natural history of the disease, and the worsening of a single parameter or the appearance of a second suspect features are sound criteria for reconsidering a surgical treatment [7,8].
When cystic lesion with an serous cystic neoplasm can be detected, recommendations regarding resection and long-term surveillance can focus on issues surrounding symptoms of local growth and progression because of extremely low rates for the cancer development. In cases of suspected IPMNs, MCNs, and solid pseudopapillary tumors, surgical resection should always achieve the complete removal of the tumor with appropriate lymphadenectomy and negative resection margins evaluated from an intraoperative frozen section. Parenchyma sparing pancreatectomy does not represent a safe procedure for all these PCNs because the disease is frequently presumed to be malignant; therefore, the procedure should be reserved for selected cases or for serous cystic neoplasms. Guidelines are useful for identifying patients at high risk due to their sensitivity [5,6]. However, these guidelines have the limitations with a low level of specificity because they are mostly based on retrospective series of resected PCNs. This selection bias may overestimate the overall risk of malignant progression, limiting the knowledge regarding a large proportion of PCNs under surveillance. IPMNs represent the largest proportion, as they account for at least 80% of PCNs and may progress to pancreatic cancer [9,10].
Follow-up should never be discontinued because repeated observations are crucial for risk stratification. At present, several follow-up schedules have been proposed in guidelines [5,6], but none of them have been shown to be the most cost effective. Interval surveillance after IPMN resection is recommended, as multifocal feature is relatively common and additional lesions may detect in remnant pancreas. In patients with noninvasive tumors that was completely resected, at least annual examination is encouraged. Surveillance for invasive IPMNs after resection have to perform same principle with guidelines for pancreatic ductal adenocarcinoma [11]. For MCNs except malignant component, given the nearly complete cure following resection of noninvasive lesions, continued surveillance is unnecessary in most cases. Overall surveillance of PCNs should be considered on an individual basis.
CONCLUSION
The prevalence of PCNs is relatively high in general population, increasing with age. PCNs account for different clinical features with various potential for malignant progression. Multidisciplinary approach for PCNs is essential in clinical practice. Guidelines help the identification of higher risk lesions, and provide recommendation in terms of surveillance strategies and the need for surgical resection. The surgeon can elaborate on a diagnostic hypothesis and assess malignancy risk, but the decision of surgery should be tailored to the individual patients.
Notes
Conflict of Interest
The author has no conflicts to disclose.