췌장암 수술 후 보조요법으로서 항암화학요법과 항암-방사선병용요법의 비교 : 단일기관 후향적 연구
Adjuvant Chemotherapy Versus Chemoradiation for Patients with Resected Pancreatic Adenocarcinoma: A Single-Center Retrospective Study
Article information
Abstract
본 연구는 췌장암 환자가 수술 후 보조 요법으로서 항암화학요법 또는 항암-방사선병용요법을 시행받는 경우에 있어서 치료 성적의 차이가 있는지 조사하기 위해 시행하였다. 2008년에서2012년까지 부산대학교병원에서 수술을 시행한 40명의 췌장 선암 환자들의 의무기록을 조사하였다. 근치적 목적으로 수술을 받은 환자 중 29명이 항암화학 요법 또는 항암-방사 선병용요법의 보조요법을 시행받았다. 보조요법의 시행 (p =0.025)과 완전 절제(p =0.043)가 더 긴 전체 생존기간과 연관이 있었다. 보조요법으로서 항암화학요법을 시행한 군과 항암-방사선병용요법을 시행한 군에서 전체 생존 기간은 유의하게 다르지 않았으나 보조요법을 하지 않은 군과 비교하였을 때에는 보조요법으로 항암화학요법을 시행한 군에서 유의하게 높은 전체 생존율을 보였다. 수술 후 보조요법은 췌장 선암 환자의 예후를 향상시키며, 항암화학요법이 항암-방사선 병용요법보다 양호한 결과를 보인다.
Trans Abstract
Background/Aims
This study aimed to compare the outcomes of patients who received systemic chemotherapy or chemoradiotherapy as adjuvant therapies following pancreatic adenocarcinoma resection.
Methods
We reviewed the medical records of 40 patients with locoregional pancreatic adenocarcinoma who underwent tumor resection at Pusan National University Hospital between 2008 and 2012.
Results
Twenty-nine patients were treated with adjuvant therapy comprising either systemic chemotherapy or chemoradiotherapy after curative-intent surgery. Adjuvant therapy (p=0.025) and complete resection (p=0.043) were associated with longer overall survival. There was no significant difference between chemotherapy and chemoradiotherapy in terms of extending overall survival; however, patients who received chemotherapy had significantly higher survival rates than those who received no adjuvant therapy at all (p=0.012).
Conclusions
Adjuvant therapies improve the prognoses of patients with resected pancreatic adenocarcinoma; moreover, chemotherapy produced more favorable outcomes than chemoradiotherapy.
INTRODUCTION
The 5-year relative survival rate of patients with cancer, adjusted for normal life expectancy, has increased by approximately 70% in Korea between 2010 and 2014, has also increased in the United States between 2005 and 2011. However, the 5-year relative survival rate of patients with pancreatic cancer in the United States was only 8% in the United States in 2006-2012 and 10.1% in Korea in 2010-2014 [1,2].
Cancer-specific survival rates in patients with pancreatic cancer, which is the fifth leading cause of cancer-related death in Korea, have been slow to improve. Limitations in early detection and the lack of effective therapeutic options substantially contribute to poor prognosis [3]. However, most patients who were diagnosed early and underwent complete resection still died of the disease. Therefore, it is important to improve post-resection survival as well as early detection.
Based on the results of numerous trials, several pancreatic cancer guidelines recommend adjuvant therapy for all patients with resected pancreatic cancer [4-9]. However, there is no definite standard regarding the optimal timing and duration of adjuvant therapy. There is also no established standard on the use of chemotherapy alone vs chemoradiotherapy. While some experts suggest that concurrent chemoradiotherapy would be superior to chemotherapy, the survival benefits provided by chemoradiotherapy have not been conclusively shown. The ongoing RTOG 0848 trial, which is investigating the benefits of chemoradiotherapy when administered in sequence with systemic chemotherapy will likely provide critical data in this regard. In this context, our study aimed to compare the effectiveness of systemic chemotherapy versus chemoradiotherapy in the adjuvant setting.
METHODS
1. Patients
We reviewed the medical records of 40 patients with pancreatic adenocarcinoma who underwent curative-intent resection at Pusan National University Hospital between November 2008 and December 2012. The tumor-nodemetastasis classification was used to stage the disease at the time of diagnosis [10]. Resectability was determined based on the involvement of adjacent structures and the presence of distant metastasis. The requirement for informed consent was waived owing to the study’s retrospective nature.
Patients underwent a pylorus-preserving pancreaticoduodenectomy (Whipple procedure) or distal pancreatectomy depending on the tumor’s location in the pancreas. Cases with microscopic evidence of tumor extension to within 1 mm of the surface margin of the resected specimen were classified as having a positive resection margin [11]. After recovery from surgery, patients underwent chemotherapy, chemoradiotherapy, or supportive care without cancer treatment. Patients receiving adjuvant chemotherapy were treated with one of the following: a 3-week interval of gemcitabine monotherapy (1,000 mg/m2, days 1 and 8), 4-week interval of 5-fluorouracil (5-FU) (425 mg/m2, days 1-5) with leucovorin (20 mg/m2, days 1-5), or 4-week interval of gemcitabine (1,000 mg/m2, days 1, 8, and 15) with capecitabine (830 mg/m2 twice a day, days 1-21). Chemoradiotherapy comprised one of the following therapies: a 4-week interval of 5-FU (15 mg/kg, days 1-4) with cisplatin (60 mg/m2, day 1), 2-week interval of 5-FU (500 mg/m2, days 1-3) with leucovorin (20 mg/m2, days 1-3), or gemcitabine monotherapy (40 mg/m2 twice weekly). For the therapeutic dose, 40 Gy of radiation was delivered by tomography. After completion of adjuvant therapy, abdominal computed tomography and serum CA 19-9 test were performed every 3 months. Data on tumor characteristics, complete resection, adjuvant therapy, and outcome of treatment were extracted from medical records and analyzed.
2. Statistical analysis
The Kaplan-Meier method was used to estimate diseasefree survival (DFS) and overall survival (OS); the log-rank test was used to compare DFS and OS among different groups. DFS was defined as the interval between surgery and detection of disease recurrence, while OS was defined as the interval between surgery and death of any cause. Patients who were alive at the time of the final follow-up visit (May 2017) or those lost to follow-up were censored. A multivariate Cox proportional hazards regression model was used to evaluate the correlations between adjuvant therapy and survival. p-values of <0.05 were considered statistically significant. The SPSS statistical software (version 18.0; SPSS Inc., Chicago, IL, USA) was used for all analyses.
RESULTS
1. Patient characteristics
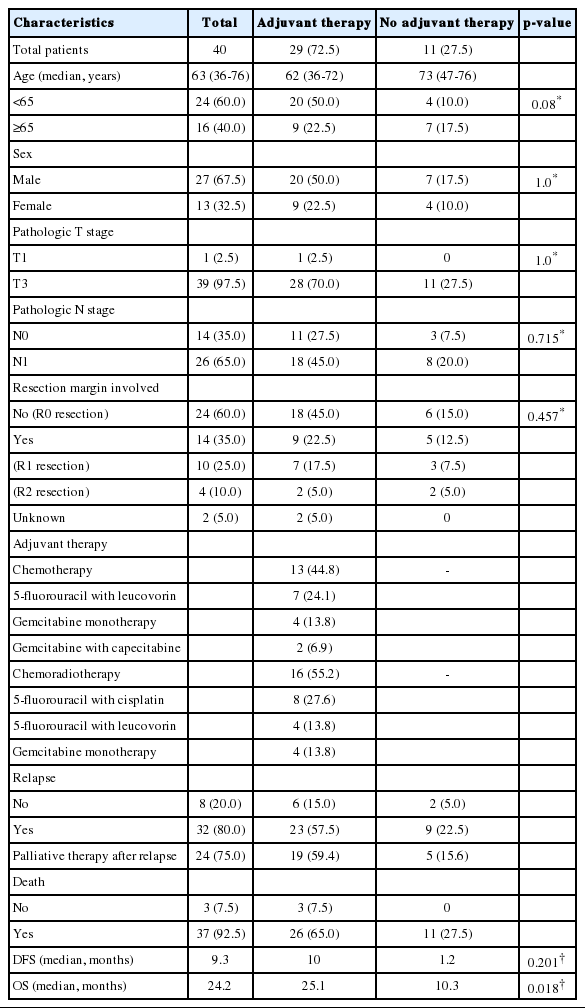
Twenty-seven men (67.5%) and 13 women (32.5%) underwent curative-intent surgery. The median age at diagnosis was 63 years (range, 36-76 years), and all patients were histologically diagnosed with pancreatic adenocarcinoma. Most patients (n=39, 97.5%) had T3 disease, while 26 (65.0%) had lymph node metastasis. Pathological reviews of the resected pancreatic masses revealed that 14 patients (35.0%) had a positive resection margin. Twenty-nine patients (72.5%) received adjuvant therapy with chemotherapy (n=13, 32.5% of all patients) or chemoradiotherapy (n=16, 40.0% of all patients). Thirty-two patients (80.0%) relapsed, 24 of whom received palliative therapy. Thirty-seven patients (92.5%) had died by May 2017 (Table 1).
2. Survival analysis
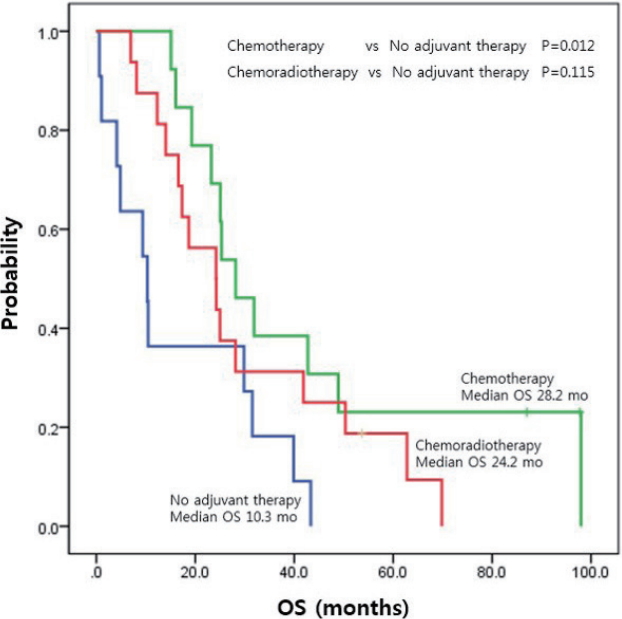
The median DFS of all patients was 9.3 months (95% confidence interval [CI], 5.8-12.8 months), while the median OS was 24.2 months (95% CI, 21.4-27.1 months). The adjuvant therapy group showed better OS (25.1 vs. 10.3 months, p =0.018) than non-adjuvant therapy group; however, there was no significant difference in DFS (10.0 vs. 1.2 months, p =0.201). With respect to the treatment method, the systemic chemotherapy group showed a higher survival rate than the non-adjuvant therapy group (median OS, 28.2 vs. 10.3 months, p =0.012). However, the chemoradiotherapy group did not show a significant improvement in OS over the non-adjuvant therapy group (median OS, 24.2 vs. 10.3 months, p =0.115) (Fig. 1).
3. Predictive factors for treatment
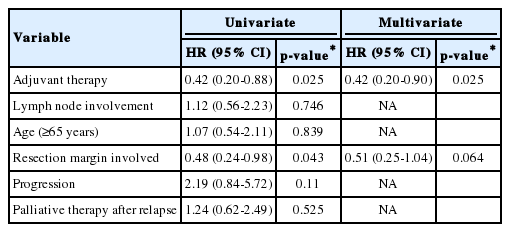
On univariate analysis, older age (≥65 years) at diagnosis, lymph node metastasis, complete resection, and adjuvant therapy were not significantly correlated with DFS. Moreover, DFS was not significantly different between the chemotherapy vs. chemoradiotherapy groups. However, receipt of adjuvant therapy (p =0.025) and complete resection (p =0.043) were significantly associated with improved OS. On multivariate logistic regression, undergoing adjuvant therapy (hazard ratio [HR], 0.42 [95% CI, 0.2-0.9], p =0.025) was the only factor predictive of improved OS (Table 2, Fig. 2).
4. Adjuvant treatment method
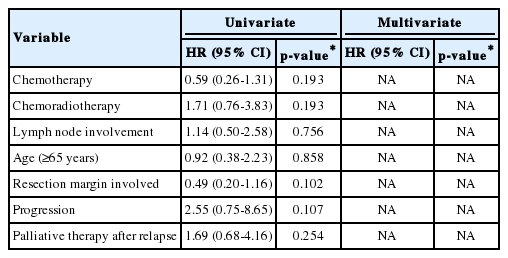
On subgroup analysis of the 29 patients who underwent adjuvant therapy, neither systemic chemotherapy nor chemoradiotherapy had a survival advantage relative to the other (DFS, 10.1 vs. 8.7 months, respectively, p =0.73; OS, 28.2 vs. 24.2 months, respectively, p =0.188). Older age (≥65 years) at diagnosis, lymph node metastasis, and complete resection did not significantly influence DFS and OS (Table 3).
5. Adverse events
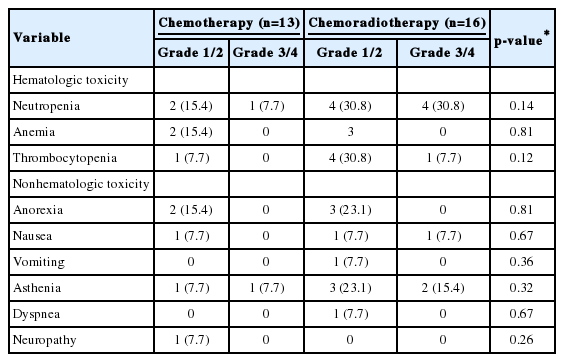
Adverse events during treatment were reported in 18 of 29 patients (62.1%) who underwent adjuvant therapy. The common adverse events were neutropenia, thrombocytopenia, anorexia, and asthenia. Grade 3/4 adverse events were more frequently reported in the chemoradiotherapy group than in the chemotherapy group; however, this difference was not significant (Table 4). Patients who experienced these toxicities were treated via conservative methods.
DISCUSSION
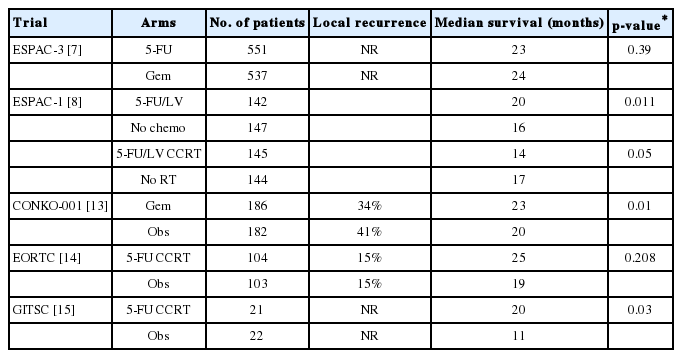
Currently, surgical resection offers the only chance for cure in patients with nonmetastatic pancreatic cancer. Only 10-20% of patients have potentially resectable disease at diagnosis [12]; however, 60-80% of patients experience recurrence despite curative surgery for pancreatic adenocarcinoma and die of metastatic disease [13,14]. For this reason, adjuvant treatment after successful tumor resection has been studied (Table 5). Oettle et al. and Neoptolemos et al. reported that adjuvant chemotherapy significantly prolonged DFS by approximately 6 months in patients with resected pancreatic cancer (CONKO-001, ESPAC-1, and ESPAC-3 trials) [8,9,12,15]. Furthermore, Kalser and Ellenberg [13] and Klinkenbijl et al. [14] reported that combining radiation and chemotherapy was effective (GITSG and EORTC40891 trials). Based on these results, adjuvant therapy is now recommended by several guidelines [4-6].
In our study, the adjuvant therapy produced better outcomes than surgery-alone. Regarding nodal status, which is one of the most important prognostic factors after complete pancreatic cancer resection, the 5-year survival rate after pancreaticoduodenectomy is only 10% for patients with node-positive disease and 30% for those with node-negative disease [16]. When our data were analyzed using a multivariate Cox regression model applying known prognostic factors including nodal status, more favorable outcomes were observed in patients who received adjuvant therapy as well.
Comparisons of the effectiveness of chemotherapy vs. chemoradiotherapy have yielded conflicting results, and no standard has been established as to which regimen should be used. Likewise, our data also did not produce any definitive conclusions; however, patients who received chemotherapy showed a significantly prolonged OS over those who did not receive any adjuvant therapy, while those who received chemoradiotherapy did not. Similar results were obtained in other large-scale studies [8,15]. Therefore, chemotherapy may be preferable over chemoradiotherapy as an adjuvant treatment. To explain the differences in OS in the absence of significant differences in DFS, further studies of factors such as regimen-associated toxicity and effect of palliative therapy after relapse are required.
Gemcitabine or 5-FU is often used when administering chemoradiotherapy; the recent RTOG 9704 trial showed no significant difference between the 2 agents [17]. According to several studies and a meta-analysis published in 2013, 5-FU and gemcitabine are considered the most effective adjuvant chemotherapy regimens for patients with resected pancreatic cancer [18,19]. Results from the recent ESPAC-4 trial indicated that adding capecitabine to gemcitabine was better than gemcitabine alone [20].
Recently, Hsieh et al. [21] reported a cohort study performed with propensity score matching in Taiwan in which 588 patients were divided into 3 groups: adjuvant chemoradiotherapy, sequential chemotherapy-radiotherapy, and chemotherapy. Their study showed that adjuvant chemoradiotherapy (HR, 0.398) and sequential chemotherapy-radiotherapy (HR, 0.307) showed improved survival over adjuvant chemotherapy alone. All treatment regimens included intensitymodulated radiotherapy, which they claimed contributed to minimizing toxicities from administering combination radiotherapy and chemotherapy and to improving survival. However, it is also a retrospective study and has limitations for a conclusion. A large-scale prospective study of this method is currently underway and we need to wait for the result.
Our study had several limitations. First, owing to its small number of patients, retrospective nature, and single-center setting, the results carried insufficient statistical power. Second, the patients’ performance statuses, tumor locations, and surgical methods were not considered; furthermore, the patients did not receive homogeneous chemotherapy and chemoradiotherapy regimens.
In conclusion, we found that adjuvant therapies had a positive effect on the prognosis of patients with resected pancreatic adenocarcinoma and that adjuvant chemotherapy produced more favorable OS outcomes than chemoradiotherapy, although this advantage was not observed in terms of DFS. Since the patient characteristics and toxicities in the chemotherapy and chemoradiotherapy groups were similar, the difference in OS may be attributable to differing patients conditions after relapse.
Notes
Conflict of Interest
The authors have no conflicts to disclose.
Acknowledgements
This work was supported by a clinical research grant from Pusan National University Hospital in 2017.