수술로 절제한 상피하 양성 십이지장 종양: 단일 기관의 증례들
Subepithelial Benign Duodenal Tumors Treated by Surgical Resection: A Case Series at A Single Institution
Article information
Trans Abstract
Background/Aims:
The incidence of duodenal tumors has increased by health surveillance. However, preoperative diagnosis of subepithelial duodenal tumors remains difficult because of the wide variety of pathologies and the location of the tumors. We analyzed endoscopic, radiological, and pathological features of subepithelial benign duodenal tumors (BDTs), which were treated by surgical resection.
Methods:
Five patients with subepithelial BDTs treated by surgical resection were analyzed retrospectively. We compared the preoperative and postoperative diagnosis and evaluated the clinical presentations, endoscopic and radiological findings, surgical treatments, pathological results, and outcomes of these patients.
Results:
All the patients underwent successful surgical resection. There were two cases of gastrointestinal stromal tumors (GISTs) treated with segmental duodenectomy, one case of carcinoid tumor treated with antrectomy, one case of gangliocytic paraganglioma treated with ampullectomy, and a lipoma removed by mass excision. The two GISTs were in the duodenal third and fourth segment close to the pancreas, and it was difficult to exclude pancreatic tumors by imaging studies. All the patients remained healthy for more than three years.
Conclusions:
Subepithelial BDTs are rare and difficult to diagnosis. Awareness and preoperative diagnosis of subepithelial BDTs can lead to minimally invasive treatment, including endoscopic or local surgical resection.
INTRODUCTION
Small intestine tumors account for about 5% of all alimentary tract tumors, with the highest proportion occurring in the duodenum[1]. Benign duodenal tumors (BDTs) account for less than 1% of small intestine tumors. The most common BDTs are adenomas, followed by other varous lesions, including gastrointestinal stromal tumors (GISTs), leiomyomas, carcinoid tumors, and lipomas[2,3]. Duodenal tumors pose diagnostic difficulties because of their rarity, nonspecific signs and symptoms, and the fact that the duodenum is usually overlooked during upper gastrointestinal endoscopy. In addition, subepithelial duodenal tumors are difficult to diagnose by endoscopic biopsy because they are located in the subepithelial layers. Due to the diagnostic difficulty and uncertain natural history of duodenal tumors, standard treatment strategies are not yet established. There are only a few reports about subepithelial BDTs[4,5]. The aim of this study was to analyze the clinical, radiological, and pathological spectra of subepithelial BDTs.
METERIALS AND METHODS
Subjects and methods
A retrospective analysis of the records of the department of surgery of Chungbuk National University Hospital, over a six-year period (2004-2010) on duodenal tumors identified five subepithelial BDTs. Clinical details on the patient’ s age, sex, and presenting symptoms were retrieved from the patient’s file. Laboratory and imaging findings were also obtained. The preoperative diagnosis was based on endoscopic or radiological features and pathological findings. The final diagnosis was based on postoperative pathological specimens. We compared the preoperative diagnosis with the final pathological diagnosis after surgical resection. The prognosis of the patients with these tumors were also analyzed.
RESULTS
The patients consisted of three males and two females with an average age of 59 years (range, 41-74). All the patients were asymptomatic, and all the tumors were detected by health surveillance. The tumors consisted of two GISTs, one lipoma, one carcinoid, and one gangliocytic paraganglioma. The preoperative diagnosis based on image findings of endoscopic or radiological studies were compatible with the final diagnosis in three out of five cases. However, preoperative and postoperative histological diagnosis was same only in the carcinoid tumor. The others were not identical to the final diagnosis due to inadequate specimens in one GIST and in the gangliocytic paraganglioma. Biopsy was not performed in one lipoma and in one GIST. All the tumors were treated with surgical resection, and all the patients remained healthy for more than three years (Table 1).
Gastrointestinal stromal tumor (GIST)
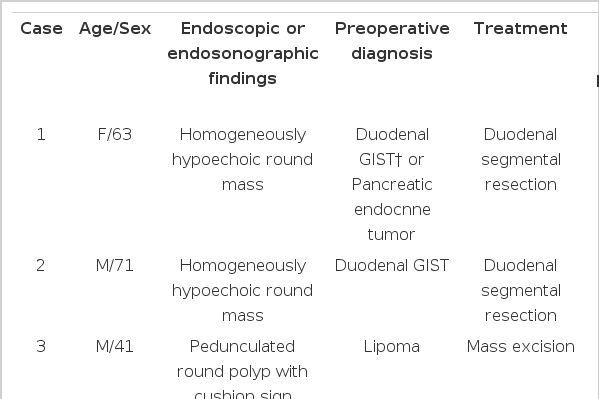
The two GISTs were located at the third and fourth portion of the duodenum. One was visible as a 4 × 4 cm sized homogenous enhancing mass, located close to the pancreas body and the small intestine on an abdominal CT scan (Fig. 1A). A PET-CT scan revealed a round mass with heterogeneous fluorodeoxyglucose (FDG) uptake(SUVmax 5.7), suggestive of malignancy (Fig. 1B). Endoscopic ultrasonography (EUS) showed a round homogenous hypoechoic mass close to the pancreas (Fig. 1C). EUS-fine needle aspiration (FNA) was nondiagnostic due to scanty cellularity, and it could not differentiate the GIST from other types of pancreatic neoplasms. The other GIST showed up as a 2.0 cm sized round enhancing mass, which was located close to the uncinate process of the pancreas on an abdominal CT scan (Fig. 2A, 2B). It was diagnosed by EUS as a stromal tumor originating from the muscle layer of the duodenum (Fig. 2C). The two GISTs were treated by duodenal segmental resection. On pathological examination, the two tumors measured 4 × 4 cm (Fig. 1D) and 2.0 × 1.5 cm, and they were located in the duodenal fourth and third portion, respectively. They were diagnosed as benign spindle cell type GISTs based on the mitotic count of <5/50 high power field (Fig. 1E). The two GISTs were positive for c-kit (Fig. 1F) and S-100 and negative for smooth muscle antigen and desmin immunostaining.

(A) An abdominal CT scan showed a round mass adjacent to the pancreas body (arrow). (B) A PET-CT scan revealed a round mass with heterogeneous FDG uptake (SUVmax 5.7), suggestive of malignancy (arrow). (C) EUS showed a homogeneous hypoechoic mass between the pancreas body and the small intestine. (D) Gross specimen of the gastrointestinal stromal tumor (GIST). (E) GIST with spindle cells and bland uniform nuclei (H-E, ×400). (F) GIST cells showing strong c-kit expression (c-kit immunostaining, ×400).
Lipoma
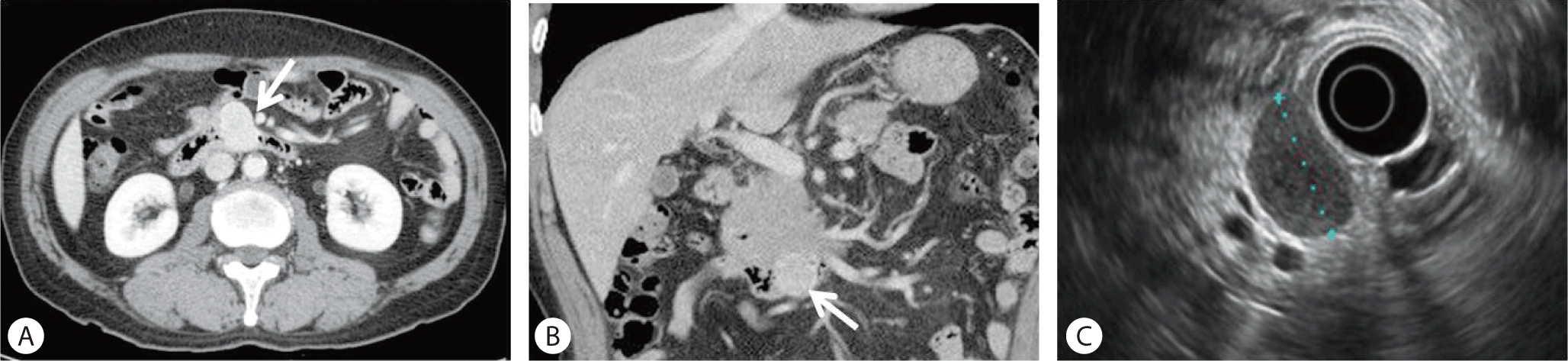
At the duodenal 2nd portion, a 2.5 × 2 cm sized pedunculated soft mass was seen in the second portion of the duodenum on gastroduodenoscopy (Fig. 3A, 3B). The preoperative diagnosis was a lipoma based on the normal mucosa covering the mass and an indentation of the mass with the biopsy forcep (cushion sign) (Fig. 3C). Mass excision was done. Pathology confirmed a duodenal lipoma arising from the duodenal second portion.
Gangliocytic paraganglioma
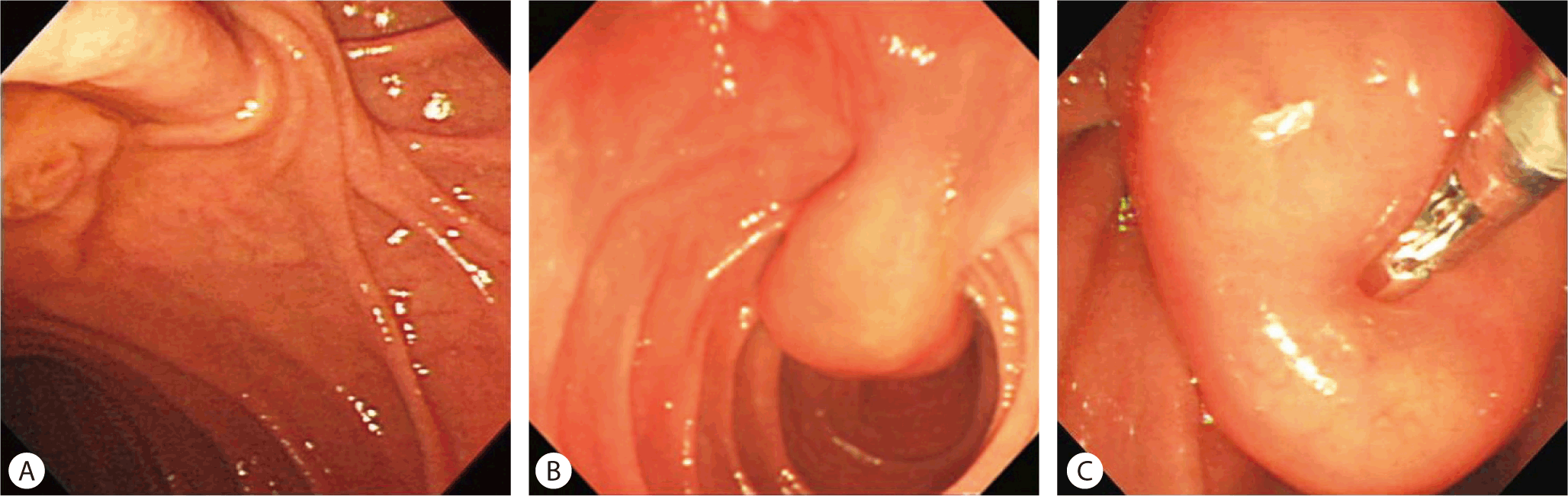
An approximately 2.0 × 1.5 cm sized round protruding mass, which was covered with normal mucosa, was noted at the major papilla (Fig. 4A). An abdominal CT scan showed an enhancing protruding ampullary mass, and a PET-CT scan revealed a round mass at the ampulla of Vater, with moderate FDG uptake (SUVmax 2.3) (Fig. 4B). After sphincterotomy, whitish fibrous tissue was revealed (Fig. 4C), and cholangiography revealed a normal biliary tree. Biopsy from the whitish lesion on the major papilla showed spindle-type cells, with positive S100 immunostaining suggestive of a neurogenic tumor. Surgical ampullectomy was done. The pathological findings showed epithelioid cells in organoid nests, as well as ganglion cells and schwannian spindle cells (Fig. 4D). This lesion was diagnosed as a gangliocytic paraganglioma, which stained positive for synaptophysin and S-100.
(A) Gastroduodenoscopy revealed a round protruding lesion 2 cm in diameter, arising in the ampulla of Vater. (B) A PET-CT scan revealed a round mass, with moderate FDG uptake (SUVmax 2.3), suggestive of a benign lesion. (C) After sphincterotomy, whitish fibrous tissue (arrow) was exposed. (D) Gangliocytic paraganglioma showing diffuse proliferation of schwannoma-like spindle cells, paraganglioma-like epithelioid cells and ganglion cells (HE, ×400).
Neuroendocrine tumor
At the duodenal bulb, a polypoid lesion about 7 mm in size was seen (Fig. 5A). An endoscopic biopsy revealed a duodenal carcinoid tumor. Carcinoid syndrome was not observed. The surgical treatment was antrectomy. The pathological diagnosis was a benign carcinoid tumor based on the cells’ characteristic round or oval nuclei and their proliferation in a trabecular and microglandular pattern without mitotic figures, granular cytoplasm, or salt and pepper nuclear chromatin, as well as chromogranin A and synaptophysin positivity (Fig. 5B, 5C).

(A) Gastroduodenoscopy showed an intraluminal submucosal polyp in the duodenal bulb. (B) Carcinoid tumor showing trabeculae and islands of tumor cells with small, round and uniform nuclei (H-E, ×400). (C) Carcinoid tumor cells showing strong expression of synaptophysin (synaptophysin immunostaining, ×400).
DISCUSSION
BDTs are uncommon and they require a different management from that of other gastrointestinal tumors. They have remained difficult to diagnose because of the vague and often asymptomatic clinical presentations. Although BDTs can occur in any portion of the duodenum, they predominately appear in the first or second portion[3,6]. With recent advances in endoscopic techniques, tumors at these locations are easily detected. However, tumors located at the third or fourth portion are much more difficult to discover.
In histopathological examinations, primary duodenal neoplasms are categorized as: epithelial tumors, mesenchymal tumors, lymphoproliferative tumors, and neuroendocrine tumors[7]. Although the incidence of subepithelial BDTs is not known, most that are discovered are GISTs and neuroendocrine tumors[7]. In this study, two GISTs, one carcinoid tumor, one gangliocytic paraganglioma, and one lipoma were found to be subepithelial BDTs.
Duodenal GISTs are rare, accounting for 1% to 4% of all GISTs[8]. Duodenal GISTs frequently involve the second portion of the duodenum, followed by the third portion, the fourth portion, and the first portion[9].
The two GISTs in this study were located at the third and fourth portion of the duodenum and had a low mitotic count. They belonged to T2N0M0G1 (AJCC stage I) and T2N0M0G1 (AJCC stage I) according to the American Joint Committee on Cancer (AJCC)[10]. The duodenal GIST presented as a pancreatic tumor because of its closeness to the pancreas[11-13]. In one of the GISTs reported here, preoperative differential diagnosis of duodenal GISTs and pancreatic tumors was impossible by abdominal CT scan, PETCT, and EUS exam. Recent study reported that GISTs with a SUVmax of over 5 in PET-CT have a high risk of malignancy[14]. However, GIST having SUVmax 5.7 in PET-CT revealed benign GIST in this study.
Duodenal lipomas are rare and usually asymptomatic. However, lipomas greater than 4 cm can produce bowel obstruction or bleeding[15]. Endoscopic findings of a cushion sign and a homogenous hyperechoic lesion originating from the submucosa with echo attenuation behind or inside the rear area are typical features of lipomas[16]. Symptomatic duodenal lipomas warrant treatment. Most cases were treated by endoscopic excision[17]. However, sessile and big lipomas need a surgical resection[18]. Our case can be managed by close observation or endoscopic excision, because it was asymptomatic and pedunculated shape. However, we performed surgical excision, since we could not obtain definite diagnosis before surgery.
Gangliocytic paraganglioma is a rare BDT, with uncertain histogenesis. By endoscopic biopsy, misdiagnosis is frequent because only one component of the tissue is obtained[19]. In our case, the preoperative diagnosis was a neurogenic tumor based on the synaptophysin-positive spindle cells in the biopsy tissue. As this lesion may present as a periampullary carcinoma, it is important to recognize and diagnose this rare, but benign, entity. Although gangliocytic paraganglioma is a benign lesion, the possibility of recurrence and metastasis exists[20]. In our study, there was one case of gangliocytic paraganglioma, which was located in the ampulla of Vater, and we performed a surgical ampullectomy.
Duodenal carcinoids are usually solitary, small, located at the first part of duodenum, and restricted to the duodenal submucosa[21]. The natural history of duodenal carcinoid tumors is poorly understood, and standard treatment strategies have yet to be established. Many studies have suggested that duodenal carcinoid tumors smaller than 2 cm in diameter and confined to the submucosal layer have limited metastatic potential[22,23]. However, one study reported the presence of regional lymph node metastases in two tumors smaller than 1 cm and limited to the submucosa[21]. Although there is a lack of consensus, the current recommendation for treatment is endoscopic removal of tumors smaller than 1.0 cm without periampullary localization or evidence of muscular propria layer invasion assessed by EUS[23]. EUS can define the size of the tumor, the level of wall invasion, and the presence of regional lymphatic metastasis[23]. The duodenal carcinoid tumor in the current study was treated by surgical resection because of limited preoperative evaluation; EUS was not available at that time in our institution. The tumor was smaller than 1 cm and was restricted to the submucosal layer. Therefore, endoscopic removal was indicated in our case.
The best treatment strategies for subepithelial BDTs have not yet been clarified. The risk of major complications with endoscopic resection of duodenal lesions is magnified compared to lesions elsewhere in the gastrointestinal tract[24]. Recent advancements in endoscopic techniques and proper preoperative diagnosis with EUS or EUS-FNA have led to the widespread use of endoscopic resection[25]. Perez et al. proposed that endoscopic treatment should be used if the tumor is less than 1 cm and surgical excision if it is more than 2 cm[3]. Surgical resection ranges from local excision to pancreaticoduodenectomy (PD), depending on the size, location, and number of lesions. Benign tumors far from the duodenal papilla can be treated with local resection or segmental duodenectomy, whereas lesions close to the pylorus must be treated with subtotal gastrectomy or antrectomy. Patients with serious damage to the papilla and biliary or pancreatic duct after tumor resection should be considered for PD[26]. Under certain circumstances, complete excision is mandatory, and PD may be considered the procedure of choice. However, PD is associated with a longer hospital stay and a higher risk of perioperative complications.
Subepithelial BDTS are rare but are detected more frequently due to the more liberal use of endoscopy. Patients may be asymptomatic or present with subtle laboratory abnormalities associated with bleeding of the lesion or partial obstruction of the ampulla. Although these lesions are benign, some have the potential to become malignant. Therefore, early diagnosis, correct treatment, and proper longterm follow-up are important. Awareness and preoperative diagnosis of subepithelial BDTs can lead to minimally invasive treatment, including endoscopic or local surgical resection.
Notes
The author has no conflicts to disclose.