내시경 역행성 담췌관조영술 시행 후 발생하는 급성 췌장염의 예방: 내시경 시술적 관점
Prevention of Post-endoscopic Retrograde Cholangiopancreatography Pancreatitis: An Endoscopic Perspective
Article information
Trans Abstract
Post-endoscopic retrograde cholangiopancreatography pancreatitis (PEP) remains to be the most common adverse event, even in experienced hands. While most of the PEP has a mild clinical course, it could be severe pancreatitis or into mortality. Recently, several endoscopic techniques, such as pancreatic stent placement, guidewire-assisted cannulation, or early precut cannulation, have been suggested as a possible techniques for the PEP prophylaxis. Since several pharmacologic agents are turned out to be non-effective or equivocal except for the rectal non-steroidal anti-inflammatory drugs which are not available in Korea, this paper will describe the general aspects of PEP and focus on the endoscopy-techniques for PEP prophylaxis.
INTRODUCTION
Endoscopic retrograde cholangiopancreatography (ERCP) has become a standard procedure for the treatment of many pancreatobiliary diseases, even though magnetic resonance cholangiopancreatography (MRCP) and endoscopic ultrasonography (EUS) have widely replaced the diagnostic aspect of ERCP. However, even in experienced hands, ERCP could be challenging and procedure-related complications might be provoked such as pancreatitis, bleeding, infection and duodenal perforation [1]. Among those complications, post-ERCP pancreatitis (PEP) is one of the most problematic complications [2]. Then, many procedure-related or pharmacological interventions have been proposed to reduce the PEP. However, several pharmacologic agents which have been proposed so far, such as corticosteroid, gabexate mesylate, nafamostat mesylate, octreotide, and somatostatin, have turned out to be non-effective or equivocal except for rectal non-steroidal anti-inflammatory drug (NSAID) [3,4]. Since rectal NSAID has not been available in South Korea until now, this paper will describe the general aspects of PEP and focus on the endoscopy-techniques for PEP prophylaxis.
1. Incidence of post-ERCP pancreatitis
Post-ERCP hyperamylasemia is quiet common. However, such a transient elevation of serum amylase does not always indicate PEP. By using the PEP definition proposed by Cotton et al. [5] in 1991, which is the presence of new pancreatic-type abdominal pain and 3 or more times the upper limits of normal occurring 24 hours after the procedure that requires at least 2 days-hospitalization, the incidence of PEP is reported to be 1-10% [5-8]. In a recently published systematic review of 108 studies including only randomized and controlled trials (RCTs), the incidence of PEP was reported to be 9.7% [9]. Furthermore, when PEP was classified into mild, moderate, and severe based on the length of hospitalization; mild (prolongation of planned hospitalization to 2-3 days), moderate (to 4-10 days), and severe (to more than 10 days, or hemorrhagic pancreatitis, phlegmon or pseudocyst, or intervention) [5], the mild, moderate, and severe pancreatitis was reported to be 5.7%, 2.6%, and 0.5%, respectively [9]. However, in high-risk patients, the incidence of PEP was increased up to 14.7% and mild, moderate, and severe pancreatitis was reported to be 8.6%, 3.9%, and 0.8%, respectively [9].
2. Mechanisms of post-ERCP pancreatitis
There are several mechanisms regarding the development of PEP [10]. First, mechanical injury to the papilla from multiple cannulation trials may lead to papillary edema and swelling as well as spasm of sphincter of Oddi, then resulting in obstruction to outflow of pancreatic juice and PEP development eventually. Second, hydrostatic injury from contrast dye or saline injection into the pancreatic duct during inadvertent pancreatic duct cannulation or sphincter manometry. Cheng et al. [11] conducted a prospective multicenter study with 15 United States centers and 1115 patients and concluded that two or more contrast injections into the pancreatic duct significantly increased the occurring of PEP. Third, thermal injury from electrosurgical current during endoscopic sphincterotomy, thermal coagulation for bleeding control, and endoscopic papillectomy [12]. This is also related to mechanical injury resulting in papillary edema from thermal burn. Lastly, translocation of intestinal flora or bacteria from contaminated duodenoscopy or accessories might lead to infection and have a role in the development of PEP [12]. Whatever the mechanism may cause to PEP, once activated, the inflammatory cascade are similar to other pancreatitis from alcohol, biliary tract disease and so on. Therefore, many strategies for each part of the mechanisms have been provided to avert PEP.
3. Risk factors for post-ERCP pancreatitis
The identification of high risk patients for PEP is important because preventive intervention could be conducted in advance, such as pancreatic duct stenting or pharmacologic prophylaxis.
Based on previous large-scale studies [13-15], the risk factors are subdivided into three categories: 1. operator-related factors; inadequate training, lack of experience, 2. patient-related factors; younger age, female sex, normal serum bilirubin, recurrent pancreatitis, prior ERCP-induced pancreatitis, sphincter of Oddi dysfunction (SOD), allergy to contrast media, pancreas divisum, 3. procedure-related factors; difficult cannulation, sphincter of Oddi manometry, precut sphincterotomy, pancreatic sphincterotomy, biliary balloon sphincteroplasty, ampullectomy, failed cannulation, cannulation time > 10 minutes, at least one pancreatic deep wire pass, two or more injections of contrast agent into the pancreatic duct, minor papilla sphincterotomy. The definition of difficult cannulation was very heterogeneous in each study. European society of gastrointestinal endoscopy (ESGE) guideline updated in 2014 specifically defines the difficult cannulation as follows: duration of > 5 minutes, > 5 attempts, or 2 pancreatic guidewire passages [16].
PREVENTION OF POST-ERCP PANCREATITIS
1. Guidewire-assisted cannulation
Mechanical injury from repeated cannulation attempts have been considered to be important mechanism for PEP development [12,17], since this could lead to papillary edema and obstruction of pancreatic ductal flow. Moreover, accidental contrast injection into pancreatic duct may lead to chemical and hydrostatic injuries as well. Therefore, a guidewire-assisted cannulation technique has been postulated to improve biliary cannulation and prevent PEP by reducing inadvertent contrast injection. Although the results of many studies are conflicting and inconclusive [18-20], several meta-analysis suggest that the guidewire-assisted cannulation reduce the risk of PEP [21,22], which has been recommended by European guideline provided by ESGE [6]. Recently in 2013, a meta-analysis of 12 randomized trials with 3450 patients also reported that the guidewire-assisted cannulation improved the biliary cannulation rate (84% vs. 77%), decreased the risk of PEP (3.5% vs 6.7%) [23], while Nakai et al. [24] reported that unintentional guidewire insertion into pancreatic duct and a small common bile duct (diameter < 9 mm) were risk factors for PEP with the use of guidewire-assisted cannulation.
Regarding pancreatic guidewire-assisted cannulation (double guidewire technique) for selective bile duct cannulation by straightening the papilla, outcomes were inconclusive and conflicting so far. However, the outcomes of two RCTs regarding the comparison of double guidewire technique and precut technique were recently reported [25,26], which showed that successful biliary cannulation were similar but, the double guidewire technique had a higher incidence of PEP (38% vs. 11%, p = 0.01) [26]. These results were reflected in the 2014 updated version of ESGE guideline, and which recommend that if this method is used, a prophylactic pancreatic stent should be placed [16].
2. Early precut sphincterotomy
Selective biliary cannulation is a pivotal element during ERCP, whereas it is unsuccessful in 5% to 10% cases with standard cannulation techniques [27]. Therefore, in such a difficult cannulation cases, precut sphincterotomy is usually performed as a rescue method and it is the essential component as expert endoscopist. However, in most cases, precut sphincterotomy was reserved for the last salvage technique when all other standard techniques have failed [28], since it has been regarded as challenging technique and independent risk factor for PEP [29,30].
However, it remains to be elusive whether the PEP is increased by the precut itself or the prolonged cannulation trials, in which multiple attempts and inadvertent pancreatic duct cannulations might be act as confounding factors [31]. ESGE guideline also comment that prolonged cannulation attempts using standard techniques may impart a risk for PEP greater than the precut sphincterotomy itself [6]. Several RCTs have conducted for comparing the early implementation of precut sphincterotomy and the repeated attempts with a standard technique so far and those studies concluded that the early implementation of precut sphincterotomy during difficult cannulation dose not increase the risk of PEP [32-35]. Moreover, in 2009, multicenter, prospective-RCT by Manes et al. [36] concluded that early precut implementation was associated with lower PEP. A recently published meta-analysis by Sundaralingam et al. [37] in 2015 reported that early implementation of precut sphincterotomy did not increase the risk of PEP compared with standard approach. Furthermore, they concluded that the risk of PEP could be reduced when it is performed by experienced expert endoscopists although further well-designed studies are necessary to confirm these findings [37].
3. Prophylactic pancreatic duct stenting
There are many prospective studies and meta-analyses suggesting pancreatic duct stent placement could be effective for the PEP prophylaxis [38-47]. As a possible explanation for this, it have been proposed that pancreatic duct stent may reduce pancreatic ductal pressure caused by papillary edema or spasm of the sphincter of Oddi [48]. A recent meta-analysis with fourteen RCTs by Mazaki et al. [47] also confirmed that prophylactic pancreatic stenting could be effective for PEP prevention after ERCP as compared with control group with relative risk (RR) of 0.39, 95% confidential interval (CI) 0.29-0.53. Furthermore, subgroup analysis according to the severity of PEP from the meta-analysis revealed that mild to moderate PEP as well as severe PEP have beneficial effect for PEP prophylaxis (RR 0.45; 95% CI 0.32-0.62 vs. RR 0.26; 95% CI 0.09-0.76) [47]. Therefore, there might be no dissent from the conclusion of prophylactic effect of pancreatic duct stent.
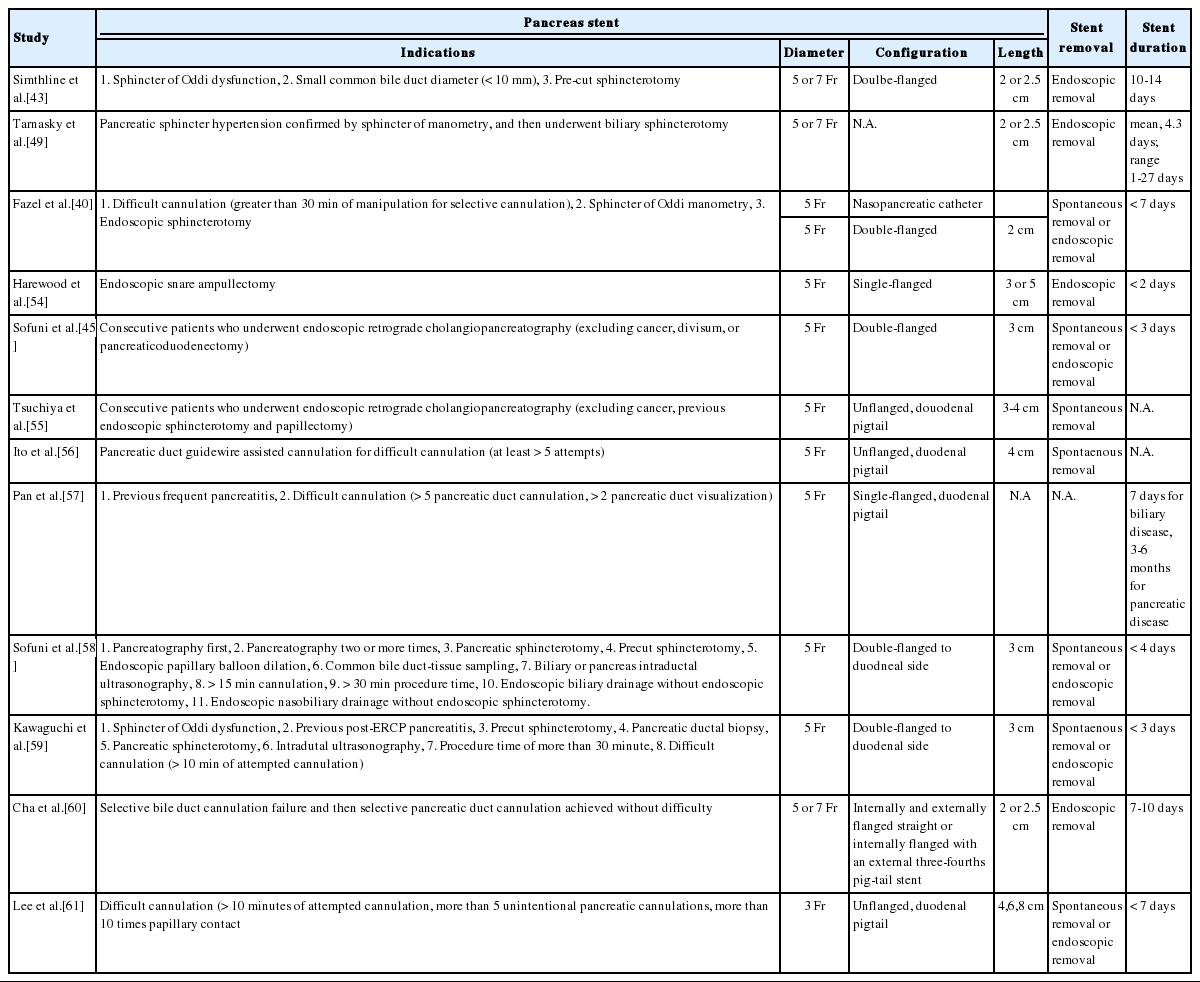
The RCTs included in the several meta-analysis, were different from each other in terms of the pancreatic stent configuration, duration in placement, and the indications of stent placement were also heterogeneous, which was summarized in Table 1. Moreover, pancreatic stent placement are not without adverse events, which have been reported to be about 5% and continuously reported since 1993 [43,49]. And these include spontaneous stent migration or occlusion, bleeding, cholangitis, cholecystitis, infection, necrosis and pancreatic duct perforation [41]. Therefore, several questions regarding pancreatic duct stent placement remains to be elusive. Who is the most suitable for prophylactic stent placement among the patients with risk factors for PEP? How long does the pancreatic stent have to be in place? Which type of pancreatic duct stent is more effective for PEP prophylaxis?
1) Who is the most suitable for prophylactic pancreatic stent placement?
Regarding the indication of prophylactic pancreatic stent placement, ESGE guideline in 2010 recommend that it should be strongly considered for high risk patients [6]. In the 2014 updated version of ESGE guideline, the high risk conditions were also stated as follows: endoscopic ampullectomy, known or suspected SOD, pancreatic sphincterotomy, precut biliary sphincterotomy, pancreatic guidewire-assisted biliary cannulation, endoscopic balloon sphincteroplasty, and presence of more than three of the risk factors [16]. Furthermore, if conventional precut technique is selected as rescue technique for selective bile duct cannulation and pancreatic duct cannulation is easily accessible, a small-diameter (3-Fr or 5-Fr) pancreatic stent is recommended to be placed and leaved in place for a minimum of 12-24 hours [16]. Kerdsirichairat et al. [50] reported that pancreatic stent insertion as a salvage measures at very early phase of the PEP within 2-48 hours also might effective for the PEP treatment.
2) Which type of pancreatic duct stent is more effective for PEP prophylaxis?
In a network meta-analysis of 6 RCTs involving 561 patients (three RCTs, 5Fr straight, flanged stent; two RCTs, 5-Fr single-pigtail, unflanged stent; three RCTs, 3-Fr single-pigtail, unflanged stents), the 5-Fr pancreatic duct stent was superior to the 3-Fr pancreatic duct stent for the PEP prevention in high-risk patients, irrespective of the configuration [51]. The probability of being the best was reported to be 50.3% for 5-Fr single-pigtail, unflanged stent, 46.5% for 5-Fr straight, flanged stents, and 3.1% for 3-Fr single-pigtail, unflanged stents [51]. A RCT at a single center showed that 5-Fr placement was easier and faster than 3-Fr stent placement, while spontaneous distal migration between the two stents was not different (5-Fr stent, 68.4%; 3-Fr stent, 75.0%; p = 0.617) [52]. On the 2014 updated version of ESGE guideline, 5-Fr pancreatic stent was more specifically recommended [16]. Furthermore, Fujisawa et al. [53] conducted single-center RCT with 240 patients to evaluate the prophylactic efficacy between short (5-Fr 3 cm) and long (5-Fr 5 cm) pancreatic stent and concluded that 5-Fr 3 cm stent was superior to 5-Fr 5 cm stent because the PEP rate was significantly lower in the short stent (5-Fr 3 cm, 2.0% vs. 5-Fr 5 cm, 8.8%, p = 0.035).
CONCLUSIONS
Several prophylactic techniques have been reviewed in this article. However, the most important thing for PEP prophylaxis is the appropriate indication for ERCP. In unnecessary or low yield cases, ERCP could be replaced with MRCP or EUS. And the identification of high risk patients for PEP is also important because preventive intervention could be conducted in advance, such as pancreatic duct stenting or guidewire-assisted cannulation. In addition, the attempts of selective cannulation should be as low as possible and in cases of difficult cannulation early precut technique may be considered as needle-knife fistulotomy is preferred in updated 2014 ESGE guidelines. Prophylactic placement of pancreatic stent with small diameter may also be considered if conventional precut is selected as rescue technique in difficult cannulation cases.
Notes
Conflict of Interest
The author has no conflicts to disclose.