분지형 점액분비성낭종 환자에서 발생한 침윤성 췌장암의 심각한 임상 경과: 증례 3예
Grave Clinical Course of Pancreatic Invasive Cancer Developed in the Patients with Branch Duct IPMN: A Report of Three Cases
Article information
Trans Abstract
Branch duct intraductal papillary mucinous neoplasms of the pancreas (BD-IPMN) without malignant features rarely developed into invasive cancer. However, invasive cancer is aggressive once an invasive change occurs. We report three cases of invasive cancers which developed in patients with BD-IPMN and they showed grave clinical courses. All patients were diagnosed with BD-IPMN < 3 cm without malignant features on imaging. Invasive cancer was detected at 2.5 years, 3.0 years, and 4.0 years after BD-IPMN detection in each patient. The intervals of invasive cancer and the last follow-up were 9 months, 3 years, and 1.5 years in the three patients, respectively. All patients were diagnosed with locally advanced pancreas invasive cancers and were treated with palliative chemotherapy or conservative management. The patients died at 3 months, 9 months, and 10 months after the diagnosis of invasive cancers, respectively. We report three cases of invasive cancer developed in BD-IPMN patients and followed fatal courses.
INTRODUCTION
Intraductal papillary mucinous neoplasms of the pancreas (IPMNs) are a spectrum of diseases ranging from adenoma, high grade dysplasia, to invasive cancer [1]. The prevalence of invasive malignancy in resected pancreatic cysts, predominantly branch duct IPMNs (BD-IPMNs) was about 15% [2]. Therefore, patients with asymptomatic BD-IPMN without “high-risk stigmata (enhancing mural nodules and main pancreatic duct (MPD) dilation more than 10 mm)” were recommended careful monitoring and surveillance [3]. However, recent studies reported that invasive cancer derived from BD-IPMN is more aggressive than the one derived from main duct IPMNs (MD-IPMNs), once an invasive change occurs [4]. There was one case report regarding unresectable invasive cancer that developed during surveillance less than 6 months after the last study in the operated BD-IPMN patient [5]. Another case of BD-IPMN measuring 2 cm without malignant features on imaging was diagnosed as an invasive cancer after resection [6]. In addition, concomitant pancreatic duct adenocarcinoma (PDAC) or other malignancies are frequent in IPMNs [7,8]. These studies suggested that subgroups of BD-IPMN were related to high malignant potentials. However, the risk factors related with fatal courses in BD-IPMN have not been identified.
In the present study, we report three cases diagnosed as locally advanced PDAC developed in the patients having BD-IPMN without malignant features on imaging; the patients followed grave clinical courses (Table 1).
CASE REPORT
Case 1
A 74-year-old man was admitted at our hospital following a five-day history of yellow skin and febrile sense. His past medical history included a pancreas cystic lesion detected two and half years ago by health surveillance. Abdominal computed tomography (CT) scan and magnetic resonance cholangiopancreatography (MRCP) revealed a 2.5 cm-sized multiseptated cystic lesion located at the body of pancreas that was suggested as BD-IPMN (Fig. 1A, B). There was no history of diabetes mellitus and hypertension. He denied any smoking or alcohol consumption. The patient had been followed every 6 or 9 months by abdominal CT and endoscopic ultrasonography (EUS) and last EUS had been performed 9 months before at another hospital (Fig. 1C). Imaging studies revealed that pancreatic cystic lesion remained of the same size without mural nodules or MPD dilation. At admission, vital signs and abdomen were normal. Laboratory tests were WBC 5,200/mm3, Hb 12.8 g/dL, platelets 215,000/mm3, AST 101 IU/L, ALT 198 IU/L, alkaline phosphatase 882 IU/L, bilirubin (total/direct) 11.6/7.6 mg/dl, amylase 9 U/L, lipase 27 U/L, and CRP 0.39 mg/dL. Serum carbonicanhydrase 19-9 (CA 19-9) was normal. An abdominal CT scan showed a 3.5 cm ill-defined low-density mass at the same site of BD-IPMN and multiple lymphadenopathies and vascular invasions to the celiac axis and hepatic artery (Fig. 1D). A positron emission tomography-computerized tomography (PET-CT) scan revealed hot uptake at the pancreatic body and peripancreatic lymph nodes. Unresectable pancreatic cancer was diagnosed by the imaging studies. Endoscopic retrograde cholangiopancreatography revealed filling defects at distal common bile duct due to pancreatic cancer invasion. Brush cytology at the bile duct revealed adenocarcinoma. We performed biliary plastic stent insertion. The patient expired 3 months afterwards due to cholangitis and liver abscess.
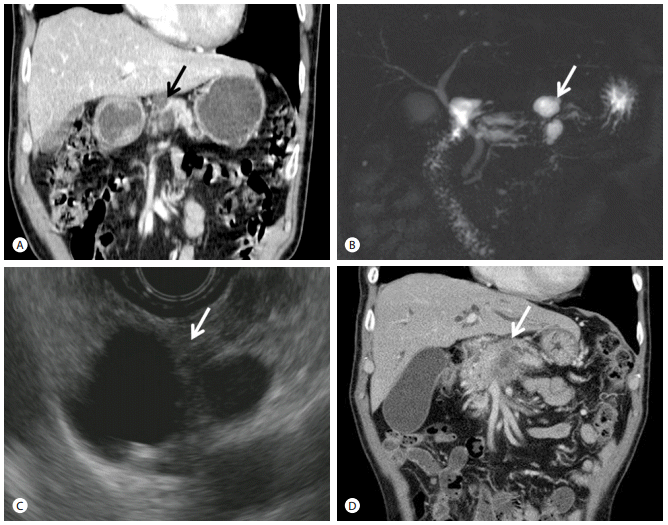
Image studies of case 1. (A, B) Abdominal CT scan (A) and MRCP (B) at 2.5 years before admission. A 2.5 cm-sized multiseptated cystic lesion(arrows) was visible at the pancreatic body. (C) EUS at 9 months before admission. Bilocular cystic lesion (arrow), measuring 1.8 cm and 1.1 cm, was seen. (D) Abdominal CT scan at admission. A 3.5 cm ill-defined low-density mass and vascular invasion to celiac axis and hepatic artery were observed (arrow). CT, computed tomography; MRCP, magnetic resonance cholangiopancreatography; EUS, endoscopic ultrasonography.
Case 2
A 62-year-old man was admitted for abdominal distension and weight loss. Pancreas cystic lesion had been diagnosed three years before. The abdominal CT scan and MRCP revealed a 2.5 cm-sized well-defined multiloculated cystic mass at the head and uncinate process of pancreas with mild MPD dilatation and pancreatic parenchymal calcification, suggested as BD-IPMN with chronic pancreatitis (Fig. 2A-C). His past medical history included diabetes mellitus. He was a current smoker (12.5 pack-years) and a chronic alcoholic. The patient had not been followed. At the present admission, his abdomen and vital signs were normal. Laboratory tests showed WBC 5,530/mm3, Hb 9.8 g/dL, platelets 225,000/mm3, AST 31 IU/L, ALT 90 IU/L, alkaline phosphatase 3,036 IU/L, albumin 3.7 g/dL, bilirubin (total/direct) 1.5/1.2 mg/dL, amylase 7 U/L, and lipase 16 U/L. CA 19-9 was normal. An abdominal CT scan showed an ill-defined 5 cm-sized low density lesion in pancreatic head with distal pancreatic duct dilatation and with invasion to distal portal vein, superior mesenteric artery, and splenic vessels (Fig. 2D). Multiple lymphadenopathies were revealed at the left gastric, porta hepatis, and peripancreatic area. EUS revealed a 4 × 2 cm-sized heterogenous hypoechoic mass in the background of cystic lesion with multiple septations at the pancreas head. Adenocarcinoma was diagnosed by EUS-fine needle aspiration (FNA). A PET-CT scan revealed hot uptake (SUV 4.3) at the pancreatic body and peripancreatic lymph nodes. Based on the image findings, unresectable PDAC was diagnosed. The patient was treated with 3 cycles of palliative gemcitabine combined with paclitaxel chemotherapy. He expired 9 months later due to cancer progression.

Image studies of case 2.(A-C) Abdominal CT scan and MRCP three years before admission. A 2.5 cm-sized multiloculated cystic mass was seen at head and uncinate process of pancreas (A and C, arrows). Main pancreatic duct dilation (arrow head) and parenchymal calcification (arrow) were revealed at pancreas body and tail (B).(D) Abdominal CT scan at admission. An ill-defined 5 cm-sized low density lesion in the pancreatic head (arrow) was observed. CT, computed tomography; MRCP, magnetic resonance cholangiopancreatography.
Case 3
A 78-year-old man was admitted for abdominal pain and weight loss. He had received transurethral resection of a bladder tumor 4 years ago and had been followed with the abdominal CT scan. The abdominal CT scan at that time had revealed a 1.5 cm-sized cystic lesion at the pancreatic body, suggested as a BD-IPMN (Fig. 3A). At 3 years before admission, the abdominal CT scan had showed BD-IPMN unchanged (Fig. 3B). However, BD-IPMN had been increased and MPD dilation by abdominal CT scan of one and half years ago (Fig. 3C). The patient had refused further evaluation and treatment until the present admission. At this admission, his abdomen and vital signs were normal. Laboratory tests showed WBC 6,870/mm3, Hb 15.3 g/dl, platelets 150,000/mm3, AST 18 IU/L, ALT 13 IU/L, alkaline phosphatase 156 IU/L, albumin 4.7 g/dL, and bilirubin 0.6 mg/dL. Serum CA 19-9 was normal. An abdominal CT scan showed a markedly increased size of ill-defined low density lesion at the pancreas body and invasion to caval, paraaortic, and peripancreatic areas and multiple lymphadenopathies (Fig. 3D). PDAC was diagnosed with EUS-FNA. He was treated with 3 cycles of palliative chemotherapy under the diagnosis of locally advanced PDAC. He expired at 10 months later due to PDAC.
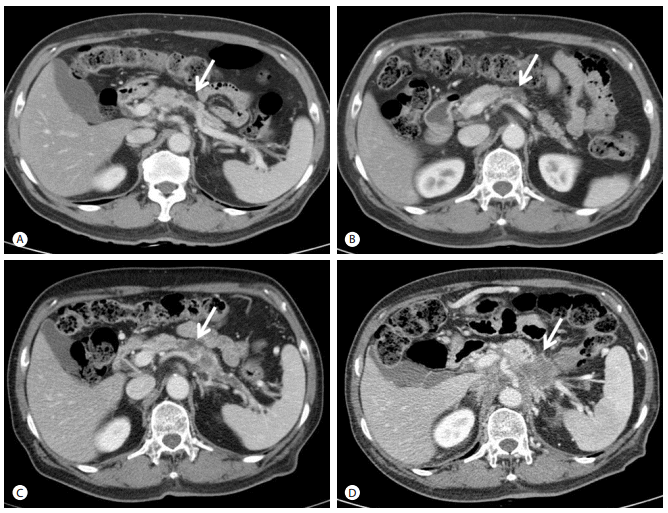
Image studies of case 3. Abdominal CT scans at 4 years (A), 3 years (B), 1.5 years (C), and 0 year (D) before admission. A 1.5 cm cystic lesion at body of pancreas was seen (A, arrow). A pancreatic cystic lesion remained unchanged (B, arrow). A pancreatic cystic lesion was increased and pancreatic duct was dilated (C, arrow). A 5.0 cm-sized of ill-defined low density lesion at body of pancreas with invasion to para-aortic and peripancreatic area was observed (D, arrow). CT, computed tomography
DISCUSSION
In this study, we reported three cases of BD-IPMN without malignant features on imaging that progressed to invasive cancers and showed grave clinical courses. BD-IPMN without malignant features usually revealed a favorable clinical course that rarely developed into invasive cancers. Also, PDAC concomitant or derived from IPMN may have more favorable biological behaviors than typical PDAC [9]. Mid-and long-term follow-up studies concluded that morphological changes are rare events in BD-IPMN [10,11]. Only 10-15% of patients showed size increase or new appearance of mural nodules and surgical resection revealed all adenoma, borderline, or carcinoma in situ [10,11].
However, a recent study reported that 38% of resected invasive cancer of BD-IPMN show metastases and worse outcomes than those derived from MD-IPMN [4]. This study suggested that invasive cancer derived from BD-IPMN showed grave course after the occurrence of an invasive morphological change. Congruently with these results, our cases are all locally advanced PDAC showing a short survival time, similarly to ordinary PDAC. There is some pathological molecular evidence that invasive cancer derived from BD-IPMN progresses to grave clinical courses with higher recurrence rates, shorter survival time, and advanced stages. First, TGF-β mRNA expression was significantly increased in BD-IPMN as compared to MD-IPMN. Immunohistochemistry also showed higher numbers of SMAD4-positive cells in the patients with carcinoma derived from BD-IPMN [4]. Transformimg growth factor (TGF)-β enhances the malignant growth of certain established epithelial tumors, promoting tumor cell proliferation, migration and epithelial-to-mesenchymal transition (EMT), which is a process by which advanced carcinoma acquires a highly invasive, undifferentiated, and metastatic phenotype. The SMAD4 is a PDAC tumor suppressor, functioning to block the progression of KRAS-initiated neoplasms, whereas in a subset of advanced tumors, intact SMAD4 facilitates EMT and TGF-beta-dependent growth [12]. EMT may play a role in IPMN tumorigenesis and metastasis. Determining TGF-β and/or SMAD4 status at the initial diagnosis may be useful for stratifying IPMN patients into treatment regimens [4]. Second, histological types vary between invasive BD-IPMN and MD-IPMN. BD-IPMNs are mostly of a gastric phenotype. Although the majority of them do not progress to invasive cancer, when they do, the carcinomas are of the tubular type [13]. The prognosis of tubular adenocarcinomas was worse, similarly to ordinary PDAC. Colloid and oncocytic carcinomas arose only from intestinal- and oncocytic-type IPMNs, respectively, and were mostly of the MD-IPMN and related with favorable prognosis. Overall survival of the patients with invasive adenocarcinomas arising from gastric-type IPMN was significantly worse than that of the patients with non-gastric-type IPMN [14]. The association between BD-IPMN and tubular adenocarcinoma seems paradoxical, since BD-IPMNs harboring gastric type epithelium are most often associated with low-grade dysplasia, absence of invasion, and fair survival rate.
It was difficult to clarify between derived from or concomitant of PDAC in BD-IPMN. Invasive IPMN was defined when the invasive solid mass extended continuously from IPMN, while concomitant cancer was considered to be the case when the invasive solid mass was located separately from the IPMN [14]. In addition to image studies, the Japan Pancreas Society defined that invasive IPMN has a histological transition between IPMN and PDAC [11]. Since our three patients with locally advanced carcinoma could not have resection, a histological transition between IPMN and PDAC could not be evaluated. However, our cases were considered as invasive-IPMN by development of PDAC at the same site of IPMN by serial images.
International consensus guidelines to manage BD-IPMN were suggested in 2012 [3]. For 1-2 cm BD-IPMN, yearly CT/magnetic resonance image for 2 years, then lengthening interval in the event of no change were recommended. For 2-3 cm BD-IPMN, EUS in 3-6 months, then lengthening the interval alternating MRI with EUS were recommended as appropriate. Recently, American Gastroenterological Association suggested that patients with pancreatic cysts < 3 cm without a solid component or a dilated pancreatic duct should undergo MRI for surveillance in 1 year and then, if there is no change in the size or characteristics of X, every 2 years for a total of 5 years [15].
Our case series were BD-IPMN < 3 cm without worrisome features (mural nodules, thickened cyst walls, dilated MPD 5-9 mm, abrupt change in MPD caliber with distal pancreatic atrophy, lymphadenopathy). Cases 2 and 3 did not follow the guidelines. Case 2 had been lost for three years and case 3 for one and half years before the diagnosis of invasive cancer. Therefore, it is important to comply with surveillance guidelines in BD-IPMN, as pancreatic cancer is a lethal disease and some portion of BD-IPMN can progress to invasive cancer. However, case 1 was followed by appropriate image studies and by proper interval satisfying the two different guidelines outlined above [3,15]. The patient had done image studies every 6 to 9 months during two and half years. However, invasive cancer developed 9 months following the last imaging. We could not find any malignant features on imaging during follow-up periods. Additional evidence is needed to predict progression; furthermore, surveillance programs should be individualized for BD-IPMN patients by new defined evidence in the near future.
We report three cases of invasive PDAC which developed in the patients having BD-IPMN without malignant features on imaging and they followed grave clinical courses.
Notes
Conflict of Interest
The author has no conflicts to disclose.
