투시영상 없이 시행한 췌장 가성낭종의 내시경초음파 유도하 배액술
Endoscopic Ultrasound-guided Pancreatic Pseudocyst Drainage without Fluoroscopy
Article information
Abstract
배경/목적
췌장 가성 낭종은 급성과 만성 췌장염의 흔한 합병증이다. 내시경 초음파를 통한 배액술은 여러단계와 여러장비들이 필요하다. 모든 병원에서 선형초음파내시경 기계 및 투시영상 검사실을 같이 갖추진 못하고 있다. 우리는 투시영상없이 초음파내시경을 통한 췌장가성낭종의 배액술의 안전성과 효율성을 확인하고자 한다.
방법
2009년 1월부터 2016년 12월까지 초음파내시경을 통해서 가성낭종의 배액술을 시행한 10명의 환자를 분석하였다. 경위적 접근법을 통하여 시행하였으며, 1개 혹은 2개의 7Fr 이중돼지꼬리 플라스틱 배액관을 사용하여 배액술을 시행하였다.
결과
기술적 성공률은 100% 이며, 임상적 성공률은 80% 였다. 2명의 환자에서는 내시경적 배액술로성공하지 못하여, 경피부배액술을 시행하였고, 수술적치료 없이 호전되었다. 3명의 환자에서는 합병증이 발생하였다(출혈, 감염, 스텐트 이탈). 평균 36.5개월을 추적관찰하였을때 가성낭종이 재발한 환자는 없었다.
결론
투시영상없이 초음파내시경을 통한 췌장 가성낭종의 배액술은 췌장가성낭종의 치료에서 안전하고, 기술적으로 가능하며, 효과적인 방법이다.
Trans Abstract
Background/Aims
Pancreatic pseudocyst is a common complication of acute and chronic pancreatitis. Endoscopy ultrasound (EUS)-guided drainage includes multiple steps and requires many resources such as a linear echoendoscope and a fluoroscopy room, which may not be available at all medical centers. We aimed to evaluate the efficacy and safety of EUS-guided pancreatic pseudocyst drainage without fluoroscopy.
Methods
This retrospective study analyzed 10 patients who had undergone EUS-guided transmural drainage of pancreatic pseudocyst without use of fluoroscopy at the Pusan National University Hospital between January 2009 and December 2016. Drainage was performed via a transgastric approach and one or two 7 Fr double-pigtail stents were inserted.
Results
The technical success rate was 100% and the clinical success rate was 80%. In two patients, clinical success was not achieved and additional percutaneous catheter drainage was done. Therefore, pseudocysts in all the patients were treated successfully without surgical drainage. However, there were three adverse events in three patients: bleeding, infection, and stent migration in each respective patient. During the median follow-up period of 36.5 months, there was no recurrence of pseudocysts in any of the patients.
Conclusions
EUS-guided transmural drainage of pseudocyst drainage without use of fluoroscopy is a technically feasible, safe, and effective procedure for the treatment of pancreatic pseudocyst.
INTRODUCTION
Pancreatic pseudocyst is a common complication of acute and chronic pancreatitis. The incidence of pancreatic pseudocyst in acute pancreatitis is 5–16%, 20-40% in chronic pancreatitis [1-3]. A majority of pseudocysts associated with acute pancreatitis resolves spontaneously within 4–6 weeks of onset [4,5]. However, pseudocysts caused by chronic pancreatitis resolve spontaneously only in about 10% of the cases [6]. The unresolved pseudocyst may be asymptomatic or may lead to epigastric pain, fever, or obstruction of the biliary tract or gastric outlet. Symptomatic and long-term unresolved pseudocyst are common indications for treatment.
In the past, surgical drainage had been the standard treatment for pancreatic pseudocysts, but endoscopic drainage is the go-to treatment these days. Since use of endoscopic ultrasound (EUS)-guidance in transmural drainage of the pancreatic pseudocysts, it has become possible to treat nonbulging pseudocysts without surgical drainage. EUS-guided transmural drainage has many advantages such as better short- and long-term prognoses, lower cost, shorter hospital stay, and improved quality of life when compared with the surgical drainage [7].
Generally, EUS-guided transmural drainage needs multiple steps and many resources such as a linear echoendoscope and a fluoroscopy room. Therefore, it would be more efficient if the number of steps for EUS-guided transmural drainage is to be minimized and use fewer resources, while maintaining the efficacy and patient safety of the procedure. In most most of previous studies, fluoroscopy was used to confirm the location of the appropriate guidewire coiling in a pseudocyst. However, a few reports have showed successful EUS-guide pseudocyst drainage without fluoroscopy [8-11]. Most hospitals do not have a special endoscopic room that can be accessible to fluoroscopy and EUS simultaneously; therefore, the EUS equipment needs to be transferred to fluoroscopy room when necessary. If the procedure could be performed without fluoroscopy, the logistics becomes simpler and both the patient and endoscopist can avoid radiation exposure. In this study, the efficacy and safety of EUS-guided transmural drainage of pancreatic pseudocyst without use of fluoroscopy were evaluated.
METHODS
Ten patients who underwent EUS-guided transmural drainage of pancreatic pseudocyst at the Pusan National University Hospital (Busan, Korea) between January 2009 and December 2016 were included. The medical records were reviewed retrospectively. A diagnosis of pancreatic pseudocyst was made in these patients by abdominal computed tomography (CT) or magnetic resonance image. The decision to perform drainage was considered when the pseudocysts caused symptoms or did not resolve after 6 to 8 weeks of follow-up. All procedures were performed by an experienced endosonoscopist (K.G.H.). The endosonocopist performed more than 200 cases of EUS-fine needle aspiration and more than 1,000 cases of endoscopic retrograde cholangiopancreatography at the entry period of this study.. None of the patients had uncorrected coagulopathy such as platelet count less than 50,000/mm3 or prothrombin time greater than 1.5 international normalized ratio. All the patients received prophylactic antibiotics.
EUS-guided transmural drainage of pancreatic pseudocyst without use of fluoroscopy was performed as follows (Fig. 1): after evaluation of the target lesion, circumambient organs, vessels, and appropriate puncture site which was less than 1 cm length between the cyst and the endoscope using EUS and endoscopy, the appropriate site for drainage was confirmed using a linear echoendoscope (GF-UCT2000; Olympus, Tokyo, Japan). A fistula between the pseudocyst and the stomach was created by introducing a 19-gauge needle for fine needle aspiration (EchoTip® Ultra Endoscopic Ultrasound Needle; Wilson-Cook, Winston-Salem, NC, USA). The cyst fluid was aspirated for analysis after the stylet was removed. A 0.035-inch guidewire (Jagwire; Boston Scientific, Natick, MA, USA) was introduced through the needle and coiled within the pseudocyst under EUS guidance, and the needle was removed after confirming that the guidewire was located within the pseudocyst. If the fistula was not sufficient for insertion of the balloon dilator, additional puncture and widening of the fistula tract was performed using a needle knife (MTW-Endoskopie, Wesel, Germany). Then, the fistula was dilated with a 6–10 mm wire-guided Hurricane balloon dilator (Microvasive; Boston Scientific). One or two 7Fr double-pigtail stents (C-Flex™ Double Pigtail Biliary Stent; Boston Scientific) were placed across the fistula at the discretion of the endoscopist. Finally, the endoscopist confirmed the location of the plastic stent(s) by using EUS.
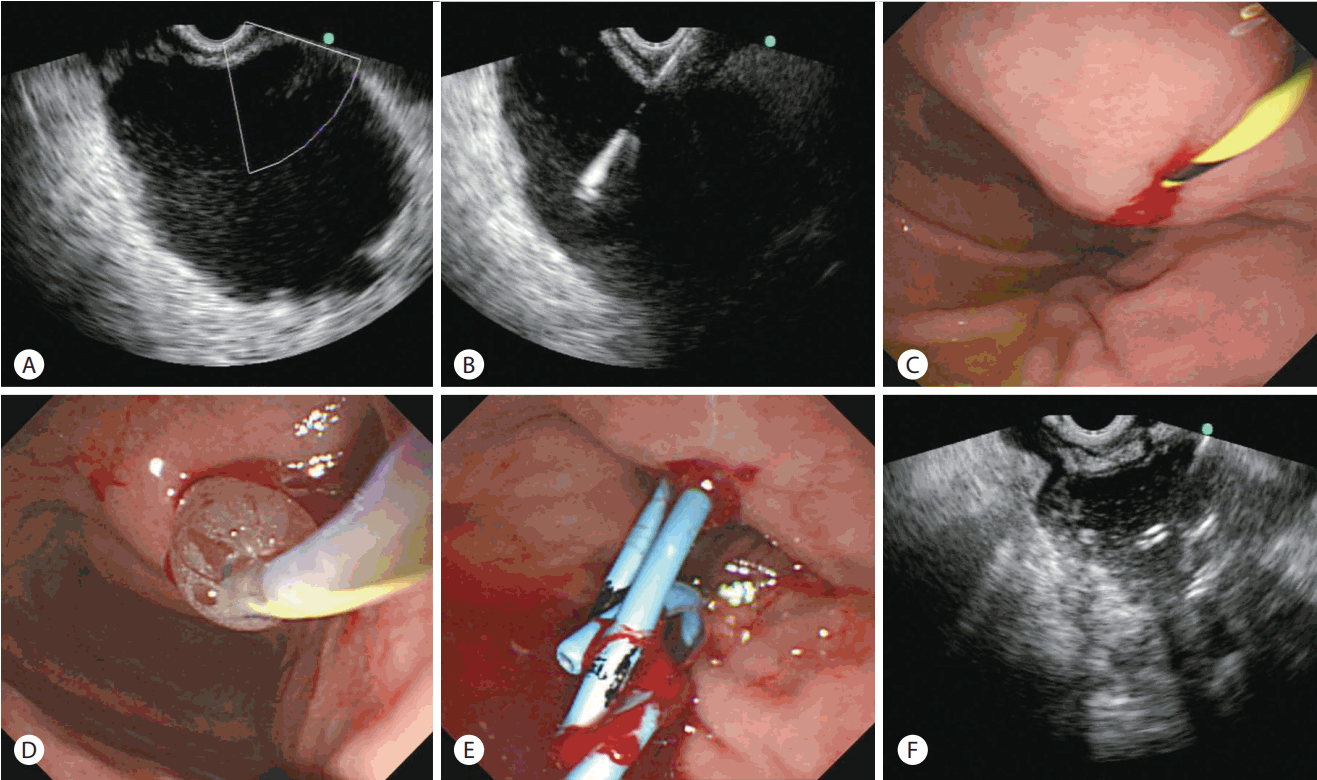
EUS-guided pseudocyst drainage without fluoroscopy. (A) After eUS and endoscopic inspection of the pseudocyst, surrounding organs, and structures, color doppler ultrasound is used to identify regional vasculature. (b) A fistula between the pseudocyst and the stomach is created by introducing a 19-gauge needle. (c) A 0.035-inch guidewire is introduced through the needle and coiled within the pseudocyst using eUS. (d) The fistula is dilated with a 6-mm balloon. (e) The balloon is exchanged off the guidewire and two 7 fr double-pigtail stents are placed across the fistula under endoscope guidance. (f) double-pigtail stents are seen inside the cyst on eUS. eUS, endoscopic ultrasound.
Technical success was defined as appropriate placement of 7Fr stent(s) across the fistula tract. Clinical success was defined as symptomatic improvement with complete disappearance or shrinking of the cyst by more than 50% of the initial measuring volume, which was assessed by CT. Procedure time was defined as the time from the start of EUS scan to appropriate placement of the plastic stent. Procedure-related adverse events were defined as newly developed adverse events after the procedure such as bleeding, pneumoperitoneum, and peritonitis [12]. Infected pseudocyst was diagnosed if purulent fluid was detected during EUS-guided drainage and/or if bacterial growth was proven in the cyst fluid cultures.
RESULTS
Ten patients (8 men and 2 women) with a median age of 53 years (range, 35–78 years) underwent EUS-guided transmural drainage of pancreatic pseudocyst without use of fluoroscopy. Clinical characteristics and outcomes are described in Table 1. The underlying causes of pseudocyst were alcohol consumption (n=6), surgery (n=3; distal pancreatectomy in 2 cases and pancreatogastrostomy in 1 case), and trauma (n=1). The median diameter of pseudocysts as revealed by CT was 8.8 cm (range, 4.5–16.5 cm), and the pseudocysts were located in the head (n=1), body (n=5), and tail (n=4) of the pancreas. The main drainage site was the posterior wall of the upper body of the stomach (7/10, 70%).
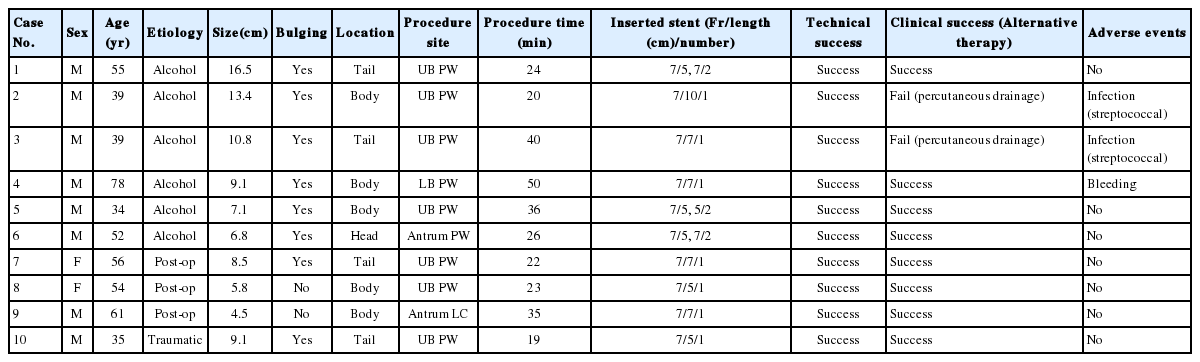
clinical characteristics and outcomes of 10 patients undergoing eUS-guided drainage without fluoroscopy
EUS-guided stent placement was successful in all the patients, and post-procedural radiography revealed that all the pigtail plastic stents were located across the fistula tract. Therefore, the technical success rate was 100%. The median procedure time of procedure was 25 minutes (range, 19–50 minutes).
One patient experienced significant bleeding during the procedure. Bleeding started through the fistula after balloon dilatation and persisted for more than 5 minutes. The bleeding site was compressed using a balloon dilator for 3 minutes, and, subsequently, the bleeding stopped. A follow-up blood count test the next day revealed that the hemoglobin concentration decreased from 13.2 g/dL to 11.0 g/dL, but there were no signs of delayed bleeding.
Clinical success was not achieved in one patient because the volume of the pseudocyst did not decrease in the follow-up CT scan, and the patient underwent an additional percutaneous drainage. During the percutaneous drainage, purulent discharge was observed and the culture of the aspirated fluid revealed growth of Streptococcus viridans. Two months after the percutaneous drainage, the pseudocyst had shrunk in volume as seen on CT scan and the stent was removed. Another patient with a huge pseudocyst in the body of the pancreas underwent EUS-guided transmural drainage using a 7Fr, 10 cm double pigtail stent. No adverse events occurred immediately after the procedure. Follow-up CT scan three weeks later revealed over 50% reduction in the volume of the pseudocyst, but the stent had migrated into the stomach. Endoscopy revealed that the stent had not completely migrated and reinsertion was performed using forceps. After repositioning of the stent, the purulent fluid was drained through the pigtail stent. Follow-up CT scan one month later revealed that the volume of pseudocyst did not decrease, and an additional percutaneous catheter drainage was performed. The culture of the drained purulent fluid revealed growth of Streptococcus constellatus. Another follow-up CT scan one month later revealed complete resolution of the pseudocyst.
As mentioned earlier, the volume of the pseudocysts did not decrease in two cases after EUS-guided transmural drainage. Therefore, the clinical success rate was 80% (8/10). When these two patients had additional percutaneous catheter drainage, the pseudocysts resolved without a need for surgical drainage. The median time til resolution of the pseudocysts that was confirmed by CT scan was 68 days (range, 60-407 days). The median time til stent removal was 106 days (range, 19-407 days), and the median follow-up period was 36.5 months (range, 1–78 months). During the follow-up, two patients experienced recurrence of pseudocysts at 26 months and 49 months after the procedure, respectively; both were caused by another episode of alcohol-induced pancreatitis. However, none of the patients needed an additional drainage procedure for the newly developed pseudocysts.
DISCUSSION
Since EUS-guided transmural drainage of pancreatic pseudocyst drainage was first reported in 1992 [13], it has become a standard treatment modality rather than percutaneous or surgical methods [7]. Although EUS-guided transmural drainage of pancreatic pseudocyst is generally performed under fluoroscopic guidance, it does not essentially require the guidance since experienced endoscopists can perform needle puncturing and stent insertion under endoscopic and EUS guidance without fluoroscopic assistance [8]. In the present study, we reviewed the data of 10 cases of EUS-guided pseudocyst drainage without fluoroscopic assistance. The technical success rate was 100% and the clinical success rate was 80%. Clinical success was not achieved in two patients and additional percutaneous catheter drainage was done. As a result, complete resolution was achieved without surgical drainage in all the patients.
Single-step EUS-guided transmural drainage was introduced in 1998, and several studies have showed that this simplified method has better success rate and efficacy than the previously used EUS-guided method [12,14,15]. Although EUS-guided transmural drainage of pancreatic pseudocyst has been simplified, it still requires several steps. During these steps, fluoroscopy is generally used to confirm that the guidewire is well positioned. With the improvement in the resolution of EUS, careful EUS inspection is capable of identifying the coiling of the guidewire and estimation of the fistula tract, which suggests that fluoroscopy does not seem to be an essential component. Non-fluoroscopy EUS-guided pseudocyst drainage was introduced in 2009, but the data on this method are limited [8-11,16].
In the present study, the rates of technical success and adverse events of EUS-guided pseudocyst drainage without fluoroscopy was 100% and 30%, respectively. These results are similar to the results of previous studies regarding EUS-guided pseudocyst drainage without fluoroscopy (a technical success rate of 80–100% and a complication rate of 0–30%) [8-11,16]. In the present study, adverse events (bleeding, infection, and stent migration) occurred in 3 patients. Because bleeding, infection, and stent migration were related to the endoscopic drainage itself, these adverse events were not associated with the procedure without fluoroscopy. In fact, clinical outcomes of the procedure without fluoroscopy are similar to those of the procedure with fluoroscopy (a technical success rate of 89–100% and benefit rate of 0–52%) [11,12,17-19]. The main benefit of fluoroscopy in the pseudocyst drainage is adjustment of the position of guidewire and the stents. therefore, the displacement of the plastic stent into the gut or pseudocyst, or outside of the pseudocyst can occur more frequently in the procedure without fluoroscopy compared with the procedure with fluoroscopy. It is important whether the inserted guidewire is coiled properly, which could be confirmed with EUS in our study. Therefore, we inserted the guidewire and then predicted the coiling state through the length of the guidewire and resistance during insertion as in other studies of non-fluoroscopy procedure [8,20,21]. According to Rana et al. [20], when the guidewire was inserted slowly under 10 cm in length, they did not experience perforation due to the guidewire. If endoscopists can be extra careful in placing the stents into the pseudocyst, the displacement of the stent during the procedure can be avoided. In fact, there was no stent displacement during the procedure in the present study. However, it is reported that the risk factors of adverse events by non-fluoroscopy procedure are thick cystic wall and a small diameter (<6 cm) of the pseudocyst [9].
Recently, there have been cases of self-expandable metal stent (SEMS) insertion for necrosectomy or drainage of material debris [22-24]. When deploying SEMS, metal stents are better seen on EUS than plastic stents; it is possible to check if the distal end of stent is expanded during EUS guidance and the proximal end is expanded during endoscopic guidance [22]. Although not performed in this study, insertion of SEMS without fluoroscopy would be available. Further studies about this procedure will be needed.
In the present study, there was no recurrence of pseudocyst due to incomplete treatment of the pseudocyst during the median follow-up period of 36.5 months. Two patients experienced recurrence of pseudocyst 26 months and 49 months later, which was caused by another episode of alcohol-induced pancreatitis; both did not need an additional procedure. Similarly, in previous studies, the rate of recurrence of pseudocyst was low (0–11.7%) and those cases improved without additional surgical treatment [8-10].
This study has several limitations. First, this study was a retrospective, single-center study, and the number of cases was too small to draw conclusions. Secondly, all EUS-guided pseudocyst drainage included in the study were performed via the transgastric route. Transduodenal route is more difficult to perform due to the angulation of the echoendoscope, and a previous study showed that the success rate of pseudocyst drainage decreased if the pseudocyst was located in the pancreatic head [12]. Therefore, the results might have been different if this study had included cases that required the transduodenal route.
In conclusion, EUS-guided transmural drainage of pancreatic pseudocyst without use of fluoroscopy showed high technical and clinical success rates, a low procedure-related adverse event and recurrence rate. Although fluoroscopic guidance is helpful, it does not seem to significantly influence clinical success in the drainage of pancreatic pseudocysts. Considering the radiation exposure and logistics of transferring equipment associated with fluoroscopy, EUS-guided transmural drainage of pancreatic pseudocyst without use of fluoroscopy may be an optimal treatment procedure in selected situations such as a large pseudocyst with thin cystic wall.
Notes
Conflict of Interest
The authors have no conflicts to disclose.
Acknowledgements
This study was supported by the Medical Research Center Program through the National Research Foundation of Korea grant funded by the Korea government (NRF-2015R1A5A2009656).