십이지장경 삽입 중 발생한 식도점막 열상으로 인해 진단된 동시성 식도암과 위암
Synchronous Esophageal and Gastric Cancer Detected by Iatrogenic Esophageal Mucosal Injury Incurred During Endoscopic Retrograde Cholangiopancreatography
Article information
Abstract
이전에 합병증 없이 내시경 역행성 담췌관 조영술(ERCP)을 여러 번 시행받은 71세 남자 환자에게 내시경 삽입 중 식도 점막 파열이 발생하였다. 점막열상은 내시경클립(endoclip)을 사용하여 성공적으로 봉합되었으나, 이 합병증으로 인해 식도암과 조기 위암이우연히 동시에 발견되었다. 십이지장경은 위장관, 특히 식도 관찰에 제약이 있기 때문에 임상적으로 중요한 병변을 놓칠 가능성이 있다. 따라서 점막 손상이나 천공을 예방하기 위해 내시경선단에 충분한 윤활제를 도포하는 것과 함께 ERCP 중 상부내시경 검사를 시행하는 것도 고려되어야 한다. 어느 환자군에서 ERCP 중 상부내시경 검사가 유용할지에 대해서는 향후 대규모 연구가 필요할 것이다.
Trans Abstract
Esophageal mucosal tear occurred during scope insertion in a 71-year-old male patient who previously underwent endoscopic retrograde cholangiopancreatography (ERCP) several times without any complications. The mucosal tear was successfully sealed with endoclips using a forward-viewing scope. However, this mishap leads to the incidental discovery of both esophageal cancer and early gastric cancer. Duodenoscope has inherent limitation in observing the gastrointestinal tract, especially the esophagus, and may miss clinically significant lesions. Therefore, in addition to applying sufficient lubricant to the scope tip and considering the possibility of anatomical variation to prevent mucosal injury or perforation, performing upper endoscopy during ERCP should be considered in a certain patient population, albeit the utility of and the population benefiting from it remains to be proven by a large-scale study.
INTRODUCTION
The duodenoscope is a side-viewing scope and the image resolution is lower than that of upper endoscope, and it is easy to miss pathologic lesions during insertion of the scope, especially the esophagus. Upper endoscopy is not a time-consuming procedure and it can easily be performed during endoscopic retrograde cholangiopancreatography (ERCP). Although performing upper endoscopy in all patients undergoing ERCP cannot be justified, it would be advisable to consider if the patient has never received upper endoscopy before, has risk factors of gastric and/or esophageal cancer, has alarm signs, or certain amount of time has passed since the last screening, etc. Herein, we report a case of a patient who was diagnosed with both esophageal cancer and early gastric cancer after experiencing the mishap of esophageal mucosal tear during ERCP.
CASE
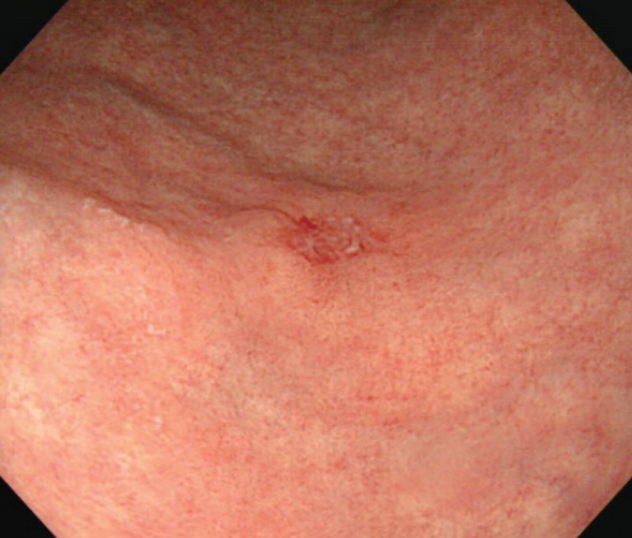
A 71-year-old man was referred to the emergency department with the impression of bile leakage after having undergone cholecystectomy for acute calculous cholecystitis. On arrival, the patient complained of severe right upper quadrant abdominal pain but his vital signs were stable except for mildly elevated body temperature (37.8℃). His laboratory test results were as follows: white blood cell count 12,240/μL (neutrophil 81.2%), total bilirubin 2.5 mg/dL, direct bilirubin 1.1 mg/dL, aspartate aminotransferase 31 IU/L, alanine aminotransferase 13 IU/L, alkaline phosphatase 242 IU/L, γ-glutamyl transpeptidase 187 IU/L, and creatinine 1.9 mg/dL. For further examination of abdominal pain and liver enzyme elevation, magnetic resonance cholangiopancreatography (MRCP) was performed instead of abdominal computed tomography (CT) scan since serum creatinine was elevated. On reconstructed MRCP image, variable sized multiple stones were observed in the dilated common bile duct (CBD) (Fig. 1). All the CBD stones could successfully be removed after three sessions of endoscopic retrograde cholangiopancreatography (ERCP) (Fig. 2). Six months later, follow-up ERCP was performed and recurred CBD stones were removed. Upon retrieval of duodenoscope, however, the screen suddenly became red when passing through the esophagus. The esophagus was examined after changing the scope to an upper endoscope, and it showed esophageal lumen stained with fresh blood along with about 4 cm long iatrogenic mucosal tear on the mid esophagus (Fig. 3A, B). The mucosal tear was sealed up by applying endoclips (Fig. 3C). During the procedure, nodular mucosal lesion was noted upstream to the mucosal tear (Fig. 3D). When biopsy was done on this lesion, it turned out to be invasive squamous cell carcinoma and therefore, esophagectomy was planned. Follow-up upper endoscopy was performed to evaluate the extent of esophageal cancer before the operation, but this time, an elevated mucosal lesion with central depression was seen on the greater curvature of gastric antrum (Fig. 4). Biopsy result of this lesion was high-grade dysplasia with suspicious foci of well-differentiated adenocarcinoma. Thus, Ivor-Lewis esophagectomy and esophageal reconstruction after wedge resection of lower body of stomach was performed to manage both lesions. After the operation, final pathology of the esophageal lesion was confirmed to be moderately differentiated invasive squamous cell carcinoma and the gastric lesion turned out to be well differentiated early gastric cancer type IIb + IIc. The patient had been well up to one year after the operation but was lost to follow-up thereafter. The patient finally returned to the outpatient clinic three and half years after surgery because of abdominal pain. On MRCP, multiple metastatic lesions were visible in the liver. Chemotherapy was considered but the patient refused further treatment and passed away 10 months later.

Multiple variable sized CBD stones are seen within the dilated bile duct on MRCP image. CBD, common bile duct; MRCP, magnetic resonance cholangiopancreatography.

CBD stones are clearly visible on fluoroscopic image (A). After three sessions of ERCP, the bile duct could be cleared of CBD stones (B). CBD, common bile duct; ERCP, endoscopic retrograde cholangiopancreatography.
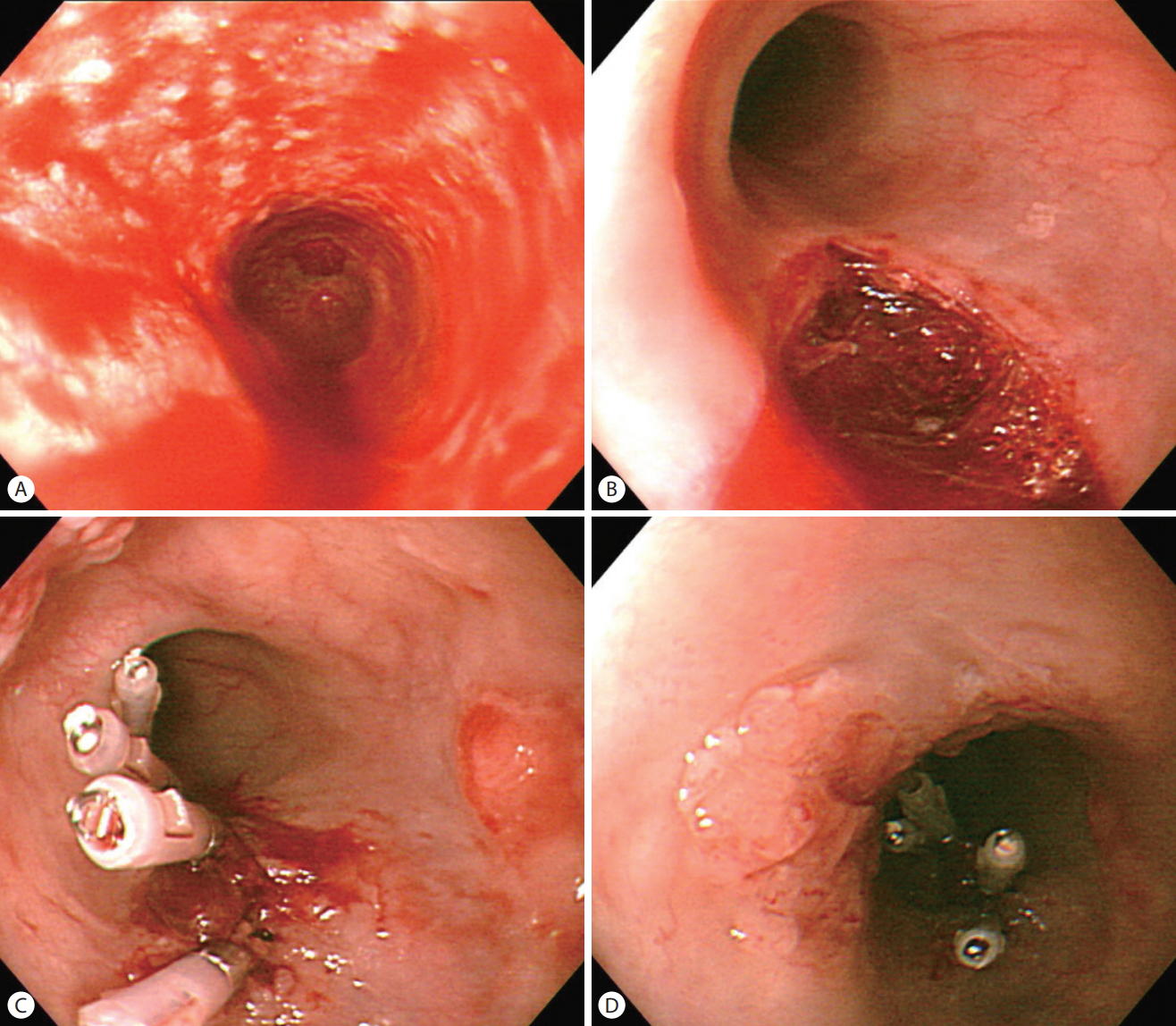
The esophageal mucosa is covered with fresh blood (A). When the blood was cleared up, about 4cm long mucosal tear could be identified on the mid esophagus (B). During mucosal tear closure with endoclips (C), nodular mucosa was observed in the vicinity of the mucosal tear which turned out to be invasive squamous carcinoma (D).
DISCUSSION
ERCP is an important modality for diagnosing and treating pancreatobiliary disorders. However, ERCP is an invasive procedure that carries 5% to 10% risk of developing complications [1]. The most common ERCP-related complications are post-ERCP pancreatitis (1.8–5.4%) and post-endoscopic sphincterotomy bleeding (1.0–2.0%) [2,3], but as with other endoscopic procedures of the gastrointestinal tract, perforation can also occur during ERCP (0.14–1.0%) [1,2]. Three types of ERCP-related perforation have been described. Type 1 is free bowel wall perforation, type 2 is retroperitoneal duodenal perforation secondary to periampullary injury, and type 3 is perforation of the CBD or pancreatic duct by the guidewire. Most of the ERCP-related perforations are type 2 with a small percentage of type 3 [4]. The risk factors are non-dilated CBD, prolonged procedure time, old age, past history of having undergone ERCP, and anatomical abnormality such as duodenal diverticulum, stenosis [1,4,5]. Type 1 perforation commonly occurs in the small bowel during scope insertion, especially in those with altered anatomy such as Billroth II anastomosis or Roux-en-Y anastomosis. However, few cases of esophageal perforations have also been reported [6,7]. Although risk factors of ERCP-related esophageal perforation have not been reported yet, it can be considered to share similar risk factors with iatrogenic mechanical trauma of esophagus during upper endoscopy such as the presence of stricture, severe inflammation, tumor, impacted food and anatomical abnormality like Zenker’s diverticulum [5]. In the present case, the patient had a very tortuous esophagus that had been pushed aside by the tortuous aorta, which could be considered a variation of an anatomical abnormality. In addition, lubrication was not applied at the scope tip during insertion of the duodenoscope. Since insertion of the duodenoscope through the esophagus occurs in a rather semi-blind fashion, aforementioned factors are likely reasons for having incurred esophageal mucosal tear. As for the location of esophageal perforation or injury, the most common site of perforation during upper endoscopy is the distal third of the esophagus because the circular layer of esophageal muscles may be thinned at this location [8]. In our case, however, the mucosal tear occurred on the mid-esophagus, likely because the greatest curve occurred on the midesophagus.
The traditional treatment of perforations has been limited to surgical management or medical observation. Recently, many of the perforations have been treated effectively and safely with endoscopy. Endoclips can be used for small perforations. Although larger perforations are usually managed surgically, endoscopic insertion of fully covered self-expandable metallic stents or self-expandable plastic stents can be considered in some cases [9]. In the present case, esophageal injury did not result in a perforation but was limited to mucosal tear which seems to have been caused by the friction between thendoscope and the mucosa during scope insertion. It could be successfully sealed by endoclips. This method has been shown to be effective even for a larger (10 cm sized) laceration in the absence of perforation [7].
Since esophageal cancer could be diagnosed because of an adverse event, this mishap could be viewed as a blessing in disguise for our patient. However, this blessing seems to have been short-lived due to the presence of synchronous gastric adenocarcinoma. The incidence of synchronous esophageal squamous cell carcinoma and gastric adenocarcinoma is rare (1.4% to 5.8%) and the overall 3-year and 5-year survival rates were 36% and 29%, respectively. For the treatment of synchronous cancer, surgery or endoscopic submucosal dissection (ESD) can be done. ESD has the advantages of being able to remove the gastric cancer endoscopically before surgical removal of esophageal cancer since the stomach can be used for reconstruction in this setting [10]. In our case, the stomach lesion seemed to be early gastric cancer and we considered ESD of this lesion prior to carrying out esophageal surgery. However, multiple small lymph nodes around the celiac trunk and left gastric artery were seen on abdominal CT scan. Although the lymph nodes might be reactive lymph nodes, the possibility of metastatic lymph node could not be ruled out. For this reason, the surgeon chose to perform wedge resection on the gastric cancer.
The patient in the present case previously underwent ERCP three times, but both the esophageal and gastric lesions were missed during examination of the mucosa upon insertion and removal of the duodenoscope. Therefore, examination of the gastrointestinal tract with a forward-viewing high-resolution scope seems to be necessary so as not to miss any clinically important lesions. A retrospective study by Thomas et al. indeed demonstrated that examination of the upper gastrointestinal tract with non-forward-viewing endoscope (endoscopic ultrasonography [EUS] and/or ERCP) missed a significant number of incidental lesions, which were mostly detected by ensuing upper endoscopic examination during the same session [11]. In a multicenter prospective cohort study, Sahakian et al. [12] also showed that upper endoscopy performed prior to EUS was beneficial in detecting clinically meaningful lesions. However, not all studies found upper endoscopy to be useful or necessary during EUS and/or ERCP. In a study by Wilcox, the gastrointestinal tract from the duodenum to esophagus was examined in all patients upon removal of the duodenoscope after completing ERCP, which was followed by additional examination with standard upper endoscope [13]. In this study, the yield of positive findings was similar between duodenoscopy and upper endoscopy, thereby claiming that examination with duodenoscope alone is sufficient. A prospective multicenter cohort study by Kim et al. [14] supports this view and states that evaluation of upper gastrointestinal tract with liner EUS alone is sufficient. These differing results seem to be due to varying indications or clinical settings, dissimilar efforts put into in attempting to identify lesions during ERCP and/or EUS, inhomogeneous timing of upper endoscopic examination, differing study design, etc. There still is no consensus or uniform standard of practice on performing upper endoscopy during ERCP and/or EUS, and it is difficult to come to a conclusion based on currently available evidence.
In conclusion, sufficient lubrication should be applied at the scope tip during insertion of the duodenoscope and the possibility of the esophagus being more tortuous than expected should be kept in mind in order to avoid complications that could arise during insertion of the duodenoscope which is done in a semi-blind fashion. Since mucosal lesions of the upper gastrointestinal tract can be easily missed during ERCP, performing upper endoscopy prior should be considered prior to ERCP in a certain patient population. A large-scale study is warranted to evaluate the true value of upper endoscopy during ERCP, select the population that would benefit the most from additional study, and determine the timing and the proper time interval for performing the examination if it proves to be meaningful.
Notes
Conflict of Interest
The authors have no conflicts to disclose.