간농양 치료 중 발생한 세프트리악손 유발성 급성 췌장염
Ceftriaxone-Induced Acute Pancreatitis in Patient with Liver Abscess
Article information
Abstract
세프트리악손은 담즙 내 침전물을 형성하여 담관염, 담낭염, 심한 경우 췌장염을 일으킬 수 있다고 알려져 있다. 70세 환자가 발열을 동반한 의식저하로 내원하여 간농양으로 진단되었고, 장기간 세프트리악손 정맥주사로 치료 중 명치 부위 통증을 호소하였다. 복부 전산화단층촬영 및 혈액검사를 통해 췌장염을 진단하였으며 세프트리악손 유발성 췌장염을 의심하여 항생제 변경 후 회복된 증례를 경험하여 이를 문헌 고찰과 함께 보고하고자 한다.
Trans Abstract
Acute pancreatitis is a rare complication in patients treated with ceftriaxone. Precipitation of ceftriaxone in the bile causes the formation of biliary sludge leading to the development of cholangitis, cholecystitis, or pancreatitis. We treated a patient with acute pancreatitis who developed this condition after the administration of ceftriaxone. A 70-year-old man presented in a drowsy state with fever. He was diagnosed with a liver abscess and treated with intravenously administrated ceftriaxone and metronidazole. He complained of dyspepsia and epigastric pain on the 25th day of ceftriaxone administration. Laboratory examination and abdominal computed tomography revealed biliary pancreatitis. Ceftriaxone-induced acute pancreatitis was suspected, and ceftriaxone administration was immediately discontinued. Two days later, serum amylase and lipase levels recovered to within reference range, and he showed rapid resolution of symptoms. We concluded that ceftriaxone results in the formation of biliary sludge and causes serious adverse events such as cholecystitis, cholangitis and biliary pancreatitis.
INTRODUCTION
Ceftriaxone is a widely used third-generation cephalosporin showing high antimicrobial activity and a long half-life. Complications of the long-term use of high dose include biliary sludge formation and cholelithiasis [1]. The risk of ceftriaxone-induced cholelithiasis is higher in patients with biliary stasis, renal failure, or fluid restriction. Most patients with ceftriaxone-induced cholelithiasis are asymptomatic. Although these complications of ceftriaxone therapy are widely known, this broad-spectrum antibiotic is not typically considered a common cause of pancreatitis. However, a few patients report serious complications such as cholecystitis and pancreatitis [2]. We report a case of a 70-year-old man who developed ceftriaxone-induced acute pancreatitis, which was treated with conservative therapy.
CASE
A 70-year-old man presented with general weakness and fever lasting several days. His medical history included hypertension, diabetes mellitus, angina, and gallstones and having undergone an open cholecystectomy. He revealed no medical history of pancreatitis or cholangitis and no allergic reaction to antibiotics. He had quit smoking and drinking alcohol 20 years ago. Upon admission, his vital sign showed blood pressure 80/50 mmHg, heart rate 106/min, respiratory rate 24/min, and body temperature 38.5℃. Laboratory tests revealed white blood cells 19,400/mm3 (normal range: 3,600–9,600/mm3) with an elevated neutrophil count (88.8%), hemoglobin 8.1 g/dL (12.9–16.9), hematocrit 24.8% (37.9–49.1), platelet 147,000/mm3 (140,000–380,000), blood urea nitrogen 49.7 mg/dL (8.0–23.0), serum creatinine 2.5 mg/dL (0.5–1.2), aspartate aminotransferase 80 U/L (<35), alanine aminotransferase 97 U/L (<40), total bilirubin 2.72 mg/dL (<1.2), lactate dehydrogenase 251 U/L (<250), alkaline phosphatase 241 U/L (35–130), γ-glutamyl transpeptidase 321 U/L (8-61), Creactive protein (CRP) 138 mg/L (<5.0), amylase 24.2 IU/L (25–125), and lipase 55.8 IU/L (7–58). Initial abdominal computed tomography (CT) showed a 2.7 cm-sized hypodense lesion in liver segments S6/5 and a periampullary diverticulum. Mild dilatation of the proximal common bile duct (CBD) (10.09 mm) and biliary sludge were observed (Fig. 1). He was diagnosed with a liver abscess and treated with intravenous administration of ceftriaxone (2 g, once a day) and metronidazole (500 mg, three times a day). Percutaneous abscess drainage was not performed because the abscess was immature. Blood cultures from two separate peripheral sites were negative. After antibiotics therapy, his symptoms and laboratory examination findings were observed to have stabilized, and follow-up CT revealed an interval decrease in the size of the abscess. He complained of dyspepsia, epigastric pain, and fever 25 days after the initial administration of ceftriaxone. Laboratory examination showed white blood cells 19,600/mm3 with an elevated neutrophil count (88.1%), blood urea nitrogen 29.7 mg/dL, serum creatinine 2.24 mg/dL, aspartate aminotransferase 28 U/L, alanine aminotransferase 30 U/L, total bilirubin 2.77 mg/dL, direct bilirubin 2.4 mg/dL, lactate dehydrogenase 352 U/L, alkaline phosphatase 376 U/L, γ-glutamyl transpeptidase 630 U/L, CRP 133.5 mg/L, triglyceride 136 mg/dL (<200), amylase 272.2 U/L, and lipase 279.9 U/L. Abdominal CT revealed dilatation and enhancement of the wall of the CBD with sludge and also showed pancreatic swelling and peripancreatic fluid collection (Fig 2). These findings were observed to be new since the previous imaging study performed 2 weeks prior. We suspected ceftriaxone-induced acute biliary pancreatitis; thus, his antibiotic was switched to ciprofloxacin. Two days after discontinuing ceftriaxone, he showed an improvement in symptoms, and laboratory examinations showed that his serum amylase and lipase levels had returned to reference range. Leukocytosis and elevated CRP were normalized 14 days later. Follow-up CT performed 16 days after discontinuing ceftriaxone showed interval improvement in pancreatitis and no sludge in the CBD (Fig. 3). He recovered uneventfully with conservative treatment and was subsequently discharged.
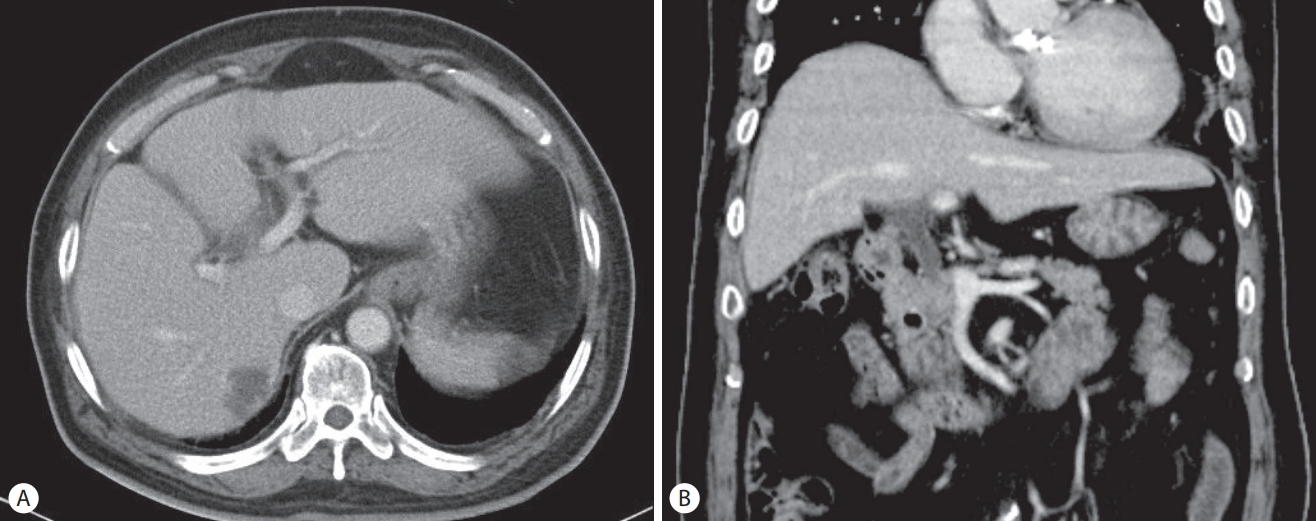
(A) Initial abdominal computed tomography scan shows a 2.7 cm-sized hypodense lesion in the S6/7 of the liver, (B) mild dilatation of the proximal common bile duct (10.09 mm) is observed without any biliary sludge.
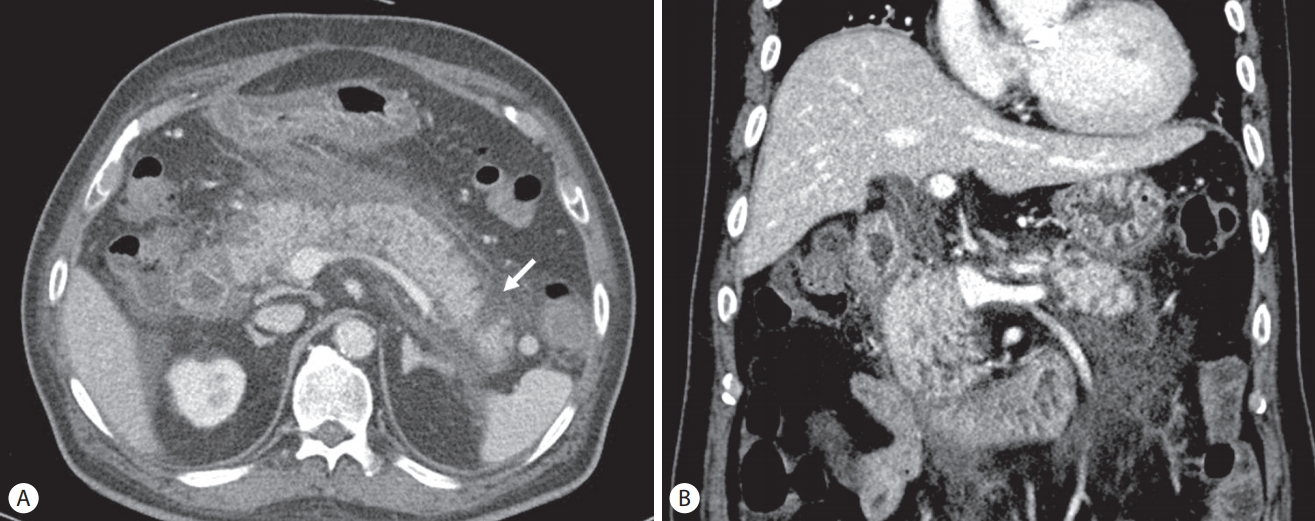
An abdominal computed tomography (CT) scan obtained 25 days after the initial administration of ceftriaxone shows: (A) Presence of pancreatic parenchymal necrosis (arrow), peripancreatic infiltration and fluid collection. (B) The diameter of the proximal common bile duct (CBD) (12.93 mm) is observed to have increased compared with the initial abdominal CT. Additionally, probable CBD sludge can be visualized.
DISCUSSION
Ceftriaxone is primarily excreted through the kidneys, and 10-20% of the drug is excreted in the bile [3]. However, a large interpatient variation has been observed in absolute bile concentrations of ceftriaoxone. Arvidsson et al. [4] observed a 3-fold interindividual variation in the biliary excretion of ceftriaxone. Furthermore, biliary excretion of ceftriaxone has been observed to show a correlation with the rate of bile acid secretion. Long-term administration of high-dose ceftriaxone intravenously has been associated with the transient formation of biliary sludge. However, this condition is usually reversible upon discontinuation of the drug [1]. The development of ceftriaxone-induced sludge of in the gallbladder of dogs and baboons was first reported in 1981. It appeared to be related to the dose of ceftriaxone and the duration of ceftriaxone administration [5]. Chemical analysis of the sludge revealed a predominance of an insoluble calcium salt of the antibiotics. Lopez et al. [6] reported that ceftriaxone-induced biliary sludge comprised 80% ceftriaxone and 20% bilirubin. Ceftriaxone is a divalent anion that gets concentrated in the canalicular bile. The concentration of ceftriaxone in the bile duct may exceed its saturation level [7]. Experiments performed in rats have shown that secretion of ceftriaxone induces the formation of an insoluble calcium-ceftriaxone complex. It has been extrapolated from known data that a dosage of >2 g/day may result in calcium-ceftriaxone precipitation [3].
Pseudolithiasis is the term used to describe imaging abnormalities observed in the gallbladder or CBD in patients treated with ceftriaxone to differentiate these ceftriaxone-induced reversible abnormalities from those related to true operable stones [8]. Pseudolithiasis was first reported by Schaad et al. [1] in 1988. Their prospective study included 37 children who were treated with ceftriaxone for various serious infections. Abdominal ultrasonography showed biliary concrements in 16 patients and three were symptomatic. These patients were treated over 4–33 days (mean 7 days) with ceftriaxone at a dose of 54-105 mg/kg/day (mean 89 mg/kg/day). Clinical symptoms and ultrasonographic abnormalities disappeared after ceftriaxone was discontinued. A double-blind controlled study performed by Heim-Duthoy et al. [8] included 28 adults who were treated with ceftriaxone for various infections. Abdominal ultrasonography showed abnormalities in six patients (21.4%) on day 14. Four of the six patients who showed ultrasonographic abnormalities were clinically asymptomatic, whereas two reported vomiting. Among eight patients treated with placebo, only one patient (12.5%) showed abnormalities on day 14. The incidence of ceftriaxone-induced pseudolithiasis is higher in children, and most patients are asymptomatic. However, ceftriaxone is known to cause severe adverse events such as cholecystitis, and pancreatitis, and even necrotizing pancreatitis, and a cholecystectomy may be required in a few patients [1]. Globally, four cases of ceftriaxone-associated pancreatitis have been reported in adults [9-12]. These patients developed acute pancreatitis after 5–10 days of treatment with ceftriaxone (2 g/day). CT showed ceftriaxone-associated pseudolithiasis with pancreatitis in 2 patients [11,12], and biliary sludge leakage from the gallbladder into the CBD and pancreatic duct causing pancreatitis in 1 [12]. Notably, four cases of ceftriaxone-induced gallbladder pseudolithiasis have been reported in Korea, however no case of ceftriaxone-associated pancreatitis has been reported [13-15].
Of note, our patient received a large dosage of ceftriaxone over several weeks, which increased his risk of ceftriaxonecalcium precipitation. Additionally, his diminished renal function and the periampullary diverticulum might have predisposed him to the development of pseudolithiasis. Calciumceftriaxone precipitations caused biliary pancreatitis and he presented with symptoms and signs such as dyspepsia, abdominal pain and fever. After discontinuation of ceftriaxone, he showed an improvement in symptoms, as well as laboratory and imaging findings.
Prolonged ceftriaxone therapy may potentially serve as risk factor for the development of pseudolithiasis and pancreatitis in patients with dehydration, periampullary diverticulum, and diminished renal function. Based on this case, we conclude that clinicians must be mindful of the possibility of ceftriaxone-induced pancreatitis in patients who present with acute abdominal pain and elevated serum amylase and lipase levels.
Notes
Conflict of Interest
The authors have no conflicts to disclose.
