담낭 소세포암에서 항암 치료 1예
Chemotherapy for Small Cell Carcinoma of Gallbladder: A Case Report
Article information
Abstract
담낭의 소세포암(SCC)은 드문 질환이다. 전이가 비교적 조기에 발생하는 경향을 보이며 예후가 좋지 않은 종양이다. 전체 담낭암의 전체 생존 기간은 약 13개월로 보고된다. 전이를 동반한 담낭암은 예후가 좋지 않으며 전체 생존 기간은 약 4개월로 보고된다. 65세 남자 환자가 담낭의 소세포암으로 진단 받고 cisplatin / etoposide 화학 요법과 방사선 요법으로 치료 받았다. 이 증례에서 3년 이상 생존한 담낭의 소세포암에 대한 결과를 보고하고자 한다.
Trans Abstract
Small cell carcinoma (SCC) of the gallbladder is a rare disease. It is an aggressive tumor that tends to metastasis early and is associated with poor prognosis. Median overall survival is reported to be approximately 13 months. Metastatic disease has a worse prognosis, and median overall survival is reported to be approximately 4 months. A 65-year-old male patient was diagnosed with the SCC of the gallbladder and was treated with cisplatin/etoposide chemotherapy followed by radiation therapy. Here, we describe the case of the SCC of the gallbladder who survived more than 3 years followed by the review of the literatures on this disease.
INTRODUCTION
Small cell carcinoma (SCC) of the gallbladder is an uncommon and a highly aggressive malignancy that was first described by Albores-Saavedra et al. [1] in 1981. The incidence of SCC among all gallbladder cancers can be estimated at about 0.5% through Surveillance, Epidemiology, and End Results (SEER) data [2]. SCC of the gallbladder is a disease process characterized by its insidious onset and advanced stage at presentation, therefore, surgical resection is impossible in most cases. Early metastasis to regional and distant lymph nodes, liver, and lungs are common with 66% of patients having evidence of metastatic disease at diagnosis [3].
Median overall survival is reported to be only 8 months [3]. Published treatment regimens for SCC of the gallbladder are highly variable, including surgical resection, radiation and a variety of chemotherapy regimens. But, no established standard care exists for treating SCC of the gallbladder.
We here report a rare case of the gallbladder SCC in a 65-year-old male. Treated with currently available modalities such as chemotherapy and radiation therapy, our patient survived 38 months from the initial time of diagnosis. To the best of our knowledge, the present case is the first case that who is diagnosed with the SCC of the gallbladder with metastasis and survived more than three years in Korea.
CASE
A 65-year-old male visited local clinic with a 3-week history of nausea, indigestion, and abdominal pain. He received esophagogastroduodenoscopy, but no significant abnormal findings were observed. In spite of taking drugs, gastrointestinal symptoms were persisted. He was referred to our hospital for further work-up. He was a non-smoker and had diabetes mellitus medical history.
Physical examination on admission revealed a blood pressure of 140/80 mmHg, heart rate of 93 beats/min, respiration rate of 20 breaths/min, and body temperature of 36.0℃. No icteric sclera was seen. The abdomen was soft, but tendered in the right upper quadrant and had an enlarged liver.
Initial laboratory findings were as follows; white blood cells 21,770/mm3 (normal reference range, 4,000-10,000/mm3), neutrophil 91.5% (40-75%), lymphocyte 3.9% (19-48%), hemoglobin 12.5 g/dL (12-16), platelets 538,000/mm3 (150,000-350,000/mm3), Erythrocyte sedimentation rate (ESR) 64 mm/h (0-20), C-reactive protein 14.13 mg/dL (0-0.5), prothrombin time 14.6 seconds (12.5-14.7), total bilirubin 0.65 mg/dL (0.3-1.2). Alkaline phosphatase 224 IU/L (30-120), gamma glutamyl transferase 56 IU/L (9-64), aspartate aminotransferase 27 IU/L (0-35), alanine aminotransferase 30 IU/L (0-35), Lactate dehydrogenase (LDH) 630 IU/L (140-271). Simple chest and plain abdominal radiography showed no specific abnormalities. For the evaluation of abdominal pain, abdominal computed tomography (CT) was performed. Abdominal CT showed 9.6×4.5 cm sized enhancing mass at the gallbladder fundus accompanied with perforation of the gallbladder fundus and pericholecystic abscess underlying xanthogranulomatous cholecystitis (Fig. 1A-C). Pancreas magnetic resonance imaging (MRI) also showed huge enhancing mass (Fig. 1D). And some amount of ascites and enhancing thickening of peritoneum suggesting peritoneal carcinomatosis were identified on abdominal CT. CA19-9 was increased with 456.9 mL/L. These findings strongly suggested a malignant tumor of the gallbladder.
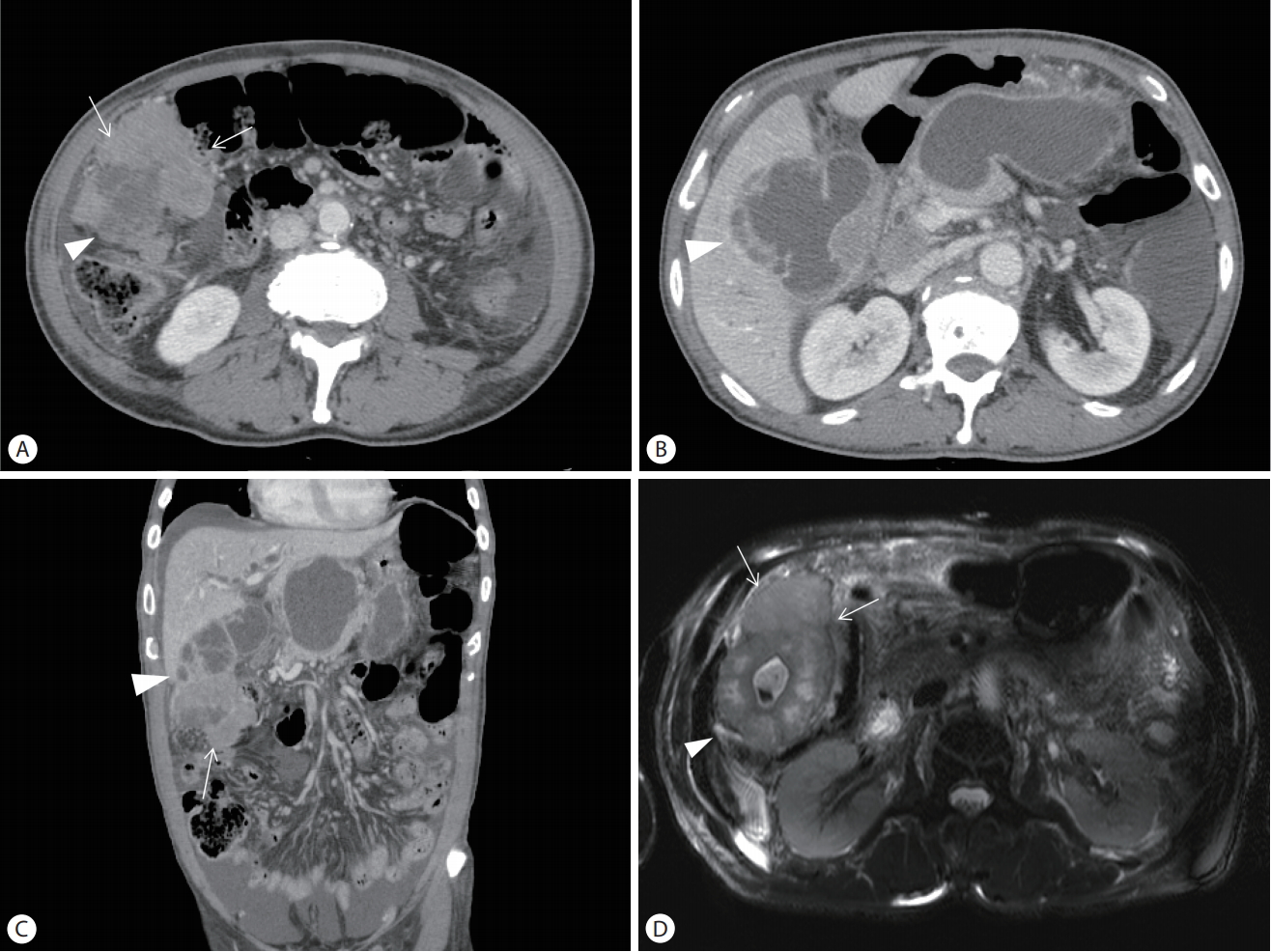
(A-C) Abdominal computed tomography and (D) Pancreas magnetic resonance imaging show 9.6×4.5 cm sized enhancing mass at the fundus of the gallbladder (white arrows) and peri-cholecystic abscess underlying xanthogranulomatous cholecystitis (arrowheads).
For histologic confirmation, ultrasound-guided biopsy was performed at the Gallbladder mass. On microscopic examination, there were uniform small round cells with high nuclear-to-cytoplasmic ratio. Immunohistochemical staining for CD56 and Ki-67 were positive, and synaptophysin and chromogranin A were negative. (Fig. 2) Upon these findings, the patient was diagnosed with the SCC of the gallbladder.

Histopathology findings show cords or festoons of uniform small round cells with hyperchromatic nuclei, inconspicuous nucleoli and scant cytoplasm (A: H&E stain, ×12.5; B: H&E stain, ×400).
After the infection control using percutaneous trans-hepatic gallbladder drainage, he was treated with etoposide (100 mg/m2) and cisplatin (100 mg/m2) beginning on day 1 of each 21-day cycle. He received three cycles of chemotherapy, but was interrupted by the onset of biliary obstruction and sepsis. Percutaneous trans-hepatic biliary drainage and biliary stent insertion were performed for relieving obstructive jaundice (Fig. 3). And he was administered with intravenous antibiotics (moxifloxacin 400 mg/day) in order to treat sepsis. However, since then, chemotherapy was discontinued due to the recurrent biliary obstruction and sepsis.
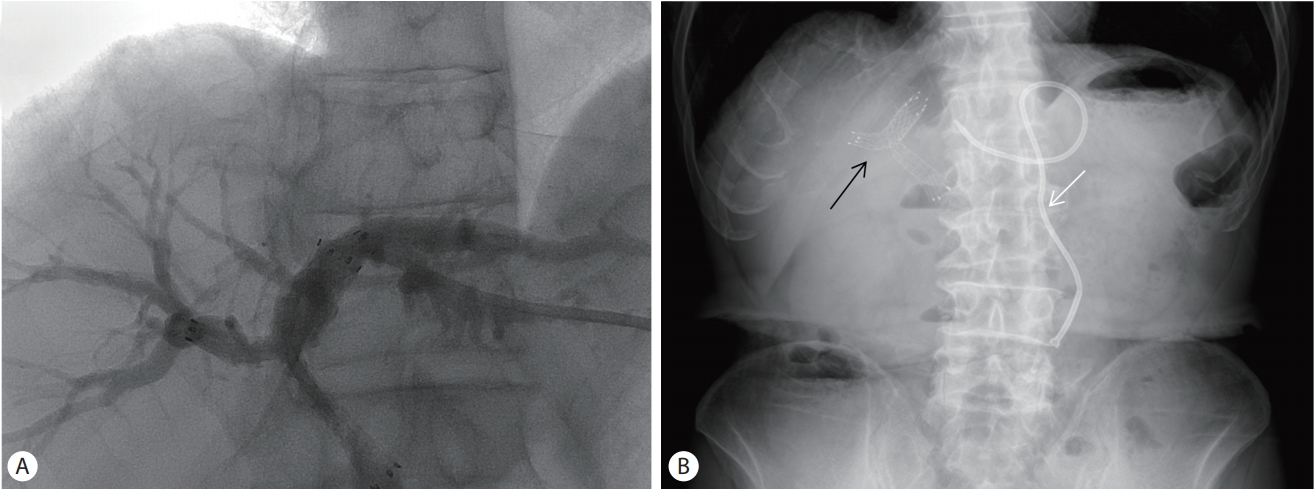
(A) Biliary stent insertion (B) Abdominal simple X-ray: Biliary stent (black arrows) and percutaneous trans-hepatic biliary drainage (white arrow) insertion.
Then palliative radiation therapy was performed because of no response to chemotherapy. As an effect of radiation therapy, the volume of the gallbladder mass was decreased, and peri-cholecystic tumor infiltration and bile duct dilatation were also improved on abdominal CT. Of the outpatient observation, the tumor progression was seen on abdominal CT, he received palliative chemotherapy changed regimen of cyclophosphamide (1,000 mg/m2), doxorubicin (45 mg/m2) and vincristine (1.4 mg/m2), and underwent palliative radiotherapy for bone metastasis. But, he experienced severe side effects of nausea, vomiting and fatigue. Therefore, the patient wanted to stop chemo-radiotherapy and only receive life-support treatment. After that, the patient developed progressive jaundice due to multiple bile duct and intrahepatic strictures, and he died 10 months after stopping the treatment and 38 months from the initial time of diagnosis.
DISCUSSION
SCC of the gallbladder is a rare disease, which has a distinct clinico-pathologic entity. Several case reports have been published; however, only 74 cases have been reported in the world literature when our case is diagnosed. Out of which, 55 were clinical cases, and 19 were autopsy cases [4,5]. This disease tends to affect an older patient population occurring at a median age of 65 years, with a female preponderance of 76% [6]. It is a disease with few well-described risk factors. Similar to adenocarcinoma of the gallbladder, there is a strong association with cholelithiasis. Interestingly, there are no neuroectodermal cells in the gallbladder mucosa [7]. This has led investigators to postulate that SCC arises from the metaplastic epithelium of the gallbladder wall [8]. This hypothesis is supported by the presence of metaplasia in the gallbladder with chronic cholecystitis [9].
Gross pathologic findings of SCC of the gallbladder include large tumor size with extensive necrosis and submucosal invasion [10]. The growth pattern is usually diffuse. Histological features show a proliferation of small round usually mixed with spindle cells both of which have scant cytoplasm, hyperchromatic nuclei and inconspicuous nucleoli under electron microscopy [7,11]. This tumor shows neuroendocrine differentiation by immunohistochemistry such as neuron-specific enolase (NSE), synaptophysin or chromogranin [7,12]. Significantly, frequent occurrences of p53 (75%), p16INK4a (33%), and K-RAS codon (17%) mutations have been identified [12].
Clinical features of SCC of the gallbladder seem to be similar to adenocarcinoma of the gallbladder such as symptoms of acute cholecystitis or right upper quadrant pain.
This tumor may be secretory with symptoms arising due to the production of biologically active peptides [13]. Conversely, most cases of this tumor are non-secretory or do not have overt clinical signs of peptides secretion as in our patient. Our patient only complained non-specific gastrointestinal symptoms such as nausea, indigestion and abdominal pain. Due to lack of systemic symptoms, SCC of the gallbladder often presents at an advanced stage with only 10% of cases confined to the gallbladder wall at the time of diagnosis [14]. Distant disease at presentation is reported as much as 75% of the cases. The most common site of metastases is lymph nodes (70%), followed by liver (64%), and lung (10%) [3].
Furthermore, given the insidious nature of the mass, diagnosis can be difficult without a histologic confirmation. Our patient also showed a large and infiltrating mass on abdominal CT and pancreas MRI. Even with these imaging findings, it is often not until the histopathologic analysis is complete that the diagnosis of a neuroendocrine tumor is reached.
Prognosis of SCC of the gallbladder remains poor. Median survival for gallbladder SCC is only 13 months. And gallbladder SCC has no survivors at 10 years according to SEER data [2]. Therapeutic options for this disease are often limited due to the advanced nature of the disease at diagnosis. To date, no standard of care exists for treating SCC of the gallbladder. Published treatment regimens for SCC of the gallbladder are highly variable, including surgical resection, radiation, and/or a variety of chemotherapy. The most commonly used chemotherapy regimens employed a platinum derivative and etoposide combination [3]. This is currently the typical treatment of SCC of the lung. Treatment of SCC of the lung currently consists of chemoradiation for limited stage disease and chemotherapy for extensive stage disease based on large clinical studies [15]. Given the similar pathology and perhaps biology of SCC of the lung and gastrointestinal tract, it is not surprising that platinum agents in combination with etoposide provide the most survival benefits.
Here, we report a rare case of the SCC of the gallbladder with metastasis diagnosed. Our patient was treated with chemotherapy and radiation therapy after the course of chemotherapy. He was not capable of reaching complete remission, but survived 28 months although previous reported median survival of the SCC of the gallbladder with metastasis was 4 months. We recommend sequential chemotherapy with platinum agents and etoposide followed by loco-regional radiation therapy. This approach can offer hope to these rare patients and contribute to increasing survival rate.
Notes
Conflict of Interest
The authors have no conflicts to disclose.