급성 담낭염과 급성 담관염을 보인 간디스토마 감염증
Clonorchis sinensis Infection Presenting as Acute Cholangitis and Acute Cholecystitis
Article information
Abstract
59세 여자가 복부 통증을 주소로 내원하였다. 복부 전산화 단층촬영에서 담관 결석이 의심되는 병변이 발견되었다. 내시경역행담췌관조영술을 하는 중에 간디스토마 성충이 배출되었다. 대변과 혈청에서 간디스토마 충란과 항체가 각각 발견되어 간디스토마 감염증으로 진단하였다. 내시경 시술과 구충제를 복용 후 증상은 서서히 호전되었다. 환자는 간디스토마 비유행지역에 거주하였고 날 것이나 덜 익힌 민물생선을 섭취한 기왕력이 없었다. 대한민국은 간디스토마 유행지역에 속하며 사람들은 조리기구 등을 통한 간접 접촉을 통해서 감염될 수 있다. 그러므로 대한민국에서 담관 질환이 있는 환자들을 진료할 때 간디스토마 감염의 가능성을 고려하여야 한다. 우리는 문헌고찰과 함께 임상 증례를 보고한다.
Trans Abstract
A 59-year-old woman presented with abdominal pain. Abdominal computerized tomography was suggestive of biliary stones. During endoscopic retrograde cholangiopancreatography, adult worms resembling Clonorchis sinensis (C. sinensis) were drained. Eggs were detected in stool using the formalin-ether concentration method and C. sinensis-specific antibody was detected in the serum. A diagnosis of C. sinensis infection was made. The symptoms of the patient gradually resolved after treatment with anti-parasite medication. The patient lived in a non-endemic region for C. sinensis infection and had no history of intake of raw or undercooked freshwater fishes. South Korea is one of the endemic countries for C. sinensis infection and people can be infected via indirect routes of transmission such as cooking utensils. Therefore, the possibility of C. sinensis infection should be considered in patients presenting with biliary diseases in South Korea. We describe the clinical findings of this case with a review of literature.
INTRODUCTION
Clonorchiasis is prevalent in South Korea, China, northern Vietnam, and far-eastern Russia [1]. In a nationwide survey conducted in 2012, it was reported that the prevalence of Clonorchis sinensis (C. sinensis ) was 1.9%, and about 932,540 residents were estimated to be infected in South Korea [2]. The egg-positive rates in the residents of five major rivers were highest in Nakdong-gang (11.7%) followed by Seomjin-gang (9.9%), Geum-gang (6.5%), Yeongsan-gang (3.1%), and Han-gang (1.0%) in 2012 [3].
Adult worms of C. sinensis can survive for a period of 20–25 years in the bile ducts, causing clonorchiasis in humans which can result in biliary stones, biliary inflammation, bile duct obstruction, liver cirrhosis, cholangiocarcinoma, and hepatic carcinoma [4]. Clonorchiasis should be suspected in people who live in or have migrated from endemic areas, have a history of consumption of raw or undercooked freshwater fish, and present with the biliary symptoms [5]. Frozen, dried, or pickled fish containing surviving metacercariae which is exported to non-endemic areas can also cause C. sinensis infection [6]. In addition, the metacercariae of C. sinensis are mucilaginous in nature and can result in indirect transmission of C. sinensis through cooking utensils [7,8]. Therefore, infection can also occur occasionally in people who have never lived in endemic areas or in those who have never consumed raw freshwater fish.
Here, we describe the clinical findings of a Korean woman who presented with abdominal pain and obstructive jaundice. The patient lived in a non-endemic area for C. sinensis infection and had no past history of fresh water fish intake. A diagnosis of clonorchiasis was made due to the presence of adult worms in bile, eggs in stool, and presence of specific antibody in the serum. We treated her with endoscopic biliary drainage and anti-helminthic medication. We report this case with a literature review.
CASE
A 59-year-old woman presented to the emergency department with a 7-day history of abdominal pain and vomiting. The pain was sudden in onset and localized in the right upper quadrant of the abdomen which worsened over a period of 8 hours, before the patient came to the hospital. The patient had diabetes mellitus and a history of canal wall up mastectomy for chronic otitis media. Her hemodynamic parameters were stable and body temperature was 37.7℃. Physical examination showed a soft and flat abdomen with normal bowel sounds, but tenderness was present in the right upper abdomen with no rebound tenderness. Murphy’s sign was positive. Laboratory data revealed a white blood cell count of 69,800/mm3 (eosinophils 25.6%), hemoglobin 12.3 g/dL, platelet 3 × 105/mm3, aspartate aminotransferase 666 IU/L, alanine aminotransferase 571 IU/L, alkaline phosphatase 571 IU/L, gammaglutamyltransferase 520 IU/L, and total bilirubin level 3.61 mg/dL. The levels of amylase, lipase, creatinine, and C-reactive protein were within normal range. Hepatitis B surface antigen, hepatitis B surface antibody, and antibody against hepatitis C virus were undetectable. Abdominal computed tomography (CT) showed high-attenuation lesions on the distal portion of the common bile duct (CBD) (Fig. 1A) and gallbladder (Fig. 1B) and diffuse dilatation of the intra-hepatic bile duct and CBD (Fig. 1C, D). These findings were suggestive of biliary tract obstruction because of stones or sludge. Endoscopic retrograde cholangiopancreatography (ERCP) revealed a round shaped filling defect in a dilated CBD (Fig. 2A). The filling defect lesions were removed using retrieval balloon and adult worms of C. sinensis were drained (Fig. 2B). Stool examination demonstrated the presence of eggs of C. sinensis showing yellow-brown ovoid operculation (Fig. 2C). Enzyme linked immunosorbent assay for C. sinensis was positive.
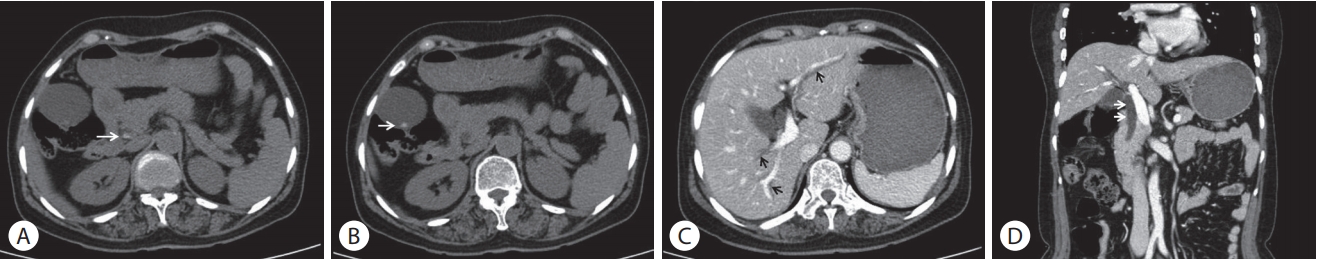
Abdominal computed tomography showed a high-attenuation lesion in the distal portion of the common bile duct (A, arrow) and in the gallbladder (B, arrow) and diffuse dilatation of the intra-hepatic bile duct (C, arrows) and common bile duct (D, arrows).
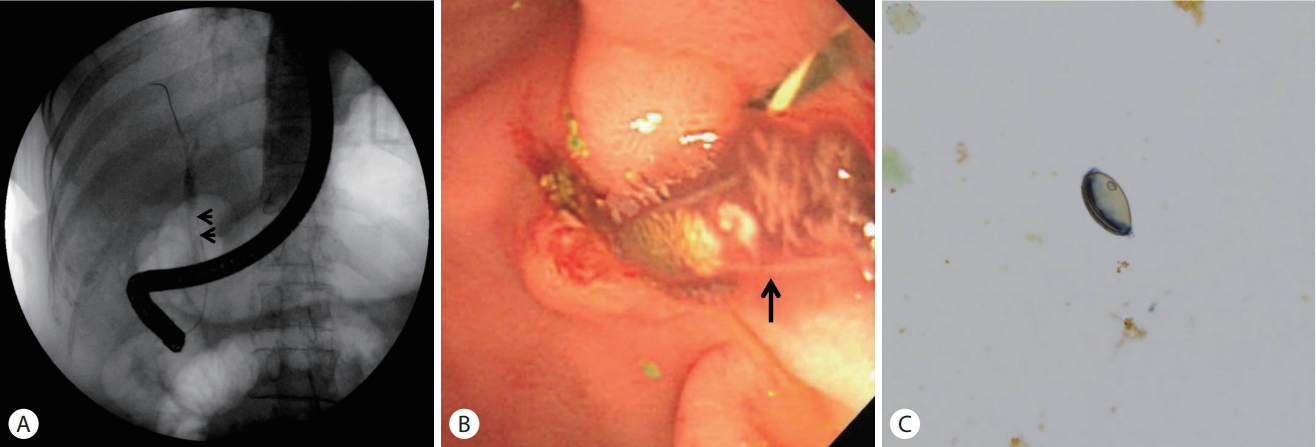
Round filling defect (A, arrows) in a dilated common bile duct on endoscopic retrograde cholangiopancreatography revealed an adult worm of Clonorchis sinensis (B, arrow). Stool examination demonstrated the eggs of Clonorchis sinensis seen as yellow-brown ovoid operculation (C, ×400).
The patient lived in a non-endemic area of clonorchiasis and had no dietary history of freshwater fish ingestion. After ERCP, the patient took three oral doses (25 mg/kg) of praziquantel for one day. Cholecystectomy was performed after 2 days. Nothing was detected in the incised gallbladder and bile. No eggs or adult worms and gallstones were found (Fig. 3A). Pathology of the resected gallbladder showed an infiltration of chronic inflammatory cells including a few eosinophils (Fig. 3B, C). The patient was discharged without any complication after surgery.
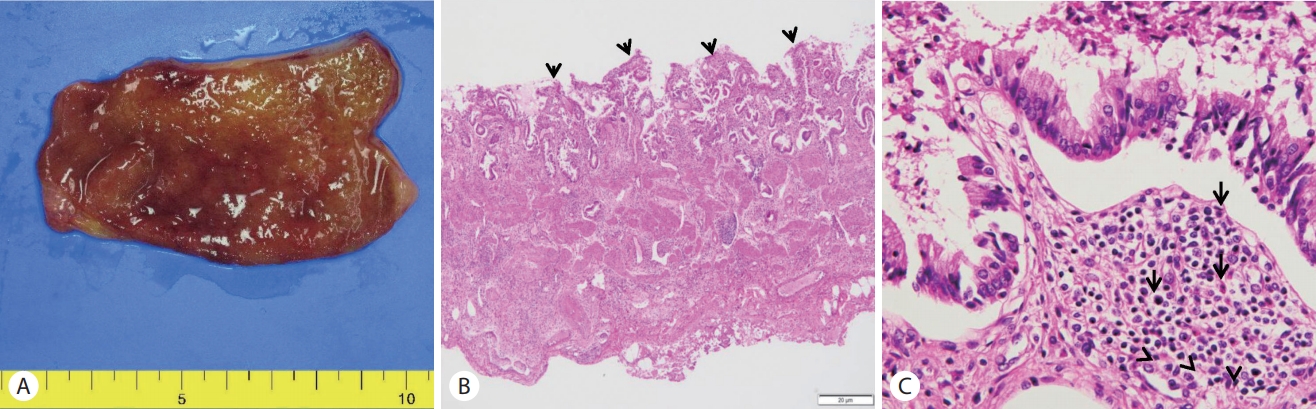
(A) Gross appearance of the resected gallbladder showed no evidence of adult worms of Clonorchis sinensis or gallstones. (B) Microscopic examination of the resected gallbladder showing the erosive mucosa and inflammatory infiltrates (arrowheads; H&E, ×40). (C) The mucosa and submucosa are infiltrated by sheets of lymphocytes and histiocytes admixed with some eosinophils (arrows; H&E, ×400).
DISCUSSION
The egg-positive rate of C. sinensis has decreased from 4.6 % in 1971 to 1.9% in 2012 in South Korea [2]. However, the egg-positive rate in people living in endemic areas reached to 12%, alongside Nakdong-gang in South Korea [3]. People who habitually eat raw or undercooked freshwater fishes and eat outside frequently have higher infection rates than those who do not [9]. The infection rate is generally higher in males than in females [9]. However, the present patient lived in a non-endemic area, and did not had any history of intake of raw or freshwater fishes, and possibly could have been infected via contaminated kitchen-wares. In a recent survey, 4.5% of the participants with C. sinensis infection did not have any history of raw fish ingestion and the discrepancy was attributed to either under-reporting or the possibility of cross-contamination from utensils [6]. In recent survey data, indirect infection rates of C. sinensis were higher in women than in men in endemic and non-endemic areas (61.5% vs. 45.0% and 75% vs. 35.7%, respectively) [10], which was attributed to the possibility of cross-contamination during the cooking process [7].
C. sinensis infection is most serious in elderly people aged 40–60 years [11], C. sinensis infection does not result in the development of a protective immunity in cases of repeated infection; rather, the cumulative worm burden increases with age among infected individuals. We speculate that our patient may have been infected with C. sinensis for a long time. C. sinensis infection can cause cholangitis, cholecystitis, liver abscess and pancreatitis. Chronic infections have been reported to cause structural abnormalities of the liver and bile ducts and to cause chronic inflammation and to be associated with the development of cholangiocarcinoma. In 2009, the International Agency for Research on Cancer classified C. sinensis as absolute carcinogen (group 1). Considering this, diagnosis and treatment of C. sinensis are important for prevention of cancer in addition to treatment of liver and biliary tract inflammation.
In this case, the patient was 58-years old and had no history of intake of raw or undercooked freshwater fish, resided in the non-endemic area. It is possible that C. sinensis have been transmitted through an unusual infection pathway rather than an usual infection pathway. Based on the above statistics, it could be possible to infect through infected chopping boards or kitchen utensils. In Korea, where the prevalence of C. sinensis is high, it should be considered that clonorchiasisinduced cholangitis and cholecystitis can occur through variable infection routes.
The present case presented as acute cholecystitis and acute cholangitis associated with C. sinensis infection. Laboratory data showed peripheral eosinophilia in addition to obstructive hepatic dysfunction. We performed ERCP and laparoscopic cholecystectomy for removal of gallstones. During ERCP, adult worms of C. sinensis were identified. Pathological examination of gallbladder suggested that C. sinensis infection occurred as a result of eosinophil infiltration; however, no eggs or adult worms were detected, which is frequently manifested in parasite infestation.We suspected that worms and eggs of C. sinensis were discharged within several days after treatment. It is difficult to diagnose clonorchiasis alone or in combination with gallstone [12,13]. Cholecystectomy in this case may not be necessary. When C. sinensis infection is suspected, follow-up imaging studies are needed to avoid unnecessary cholecystectomy.
In the present study, a diagnosis of C. sinensis infection was made due to the detection of adult worms in bile, eggs in stool, and specific antibody in the serum. Stool test for eggs of C. sinensis was performed using the formalin-ether concentration technique. This method is more sensitive in contrast to the direct fecal smear, the Kato-Katz method [14]. Therefore, it is useful for the diagnosis of light infection. ELISA test, which is considered sufficiently sensitive for the serodiagnosis of clonorchiasis, was also performed using crude extracts of C. sinensis . However, its result can be false positive in cases with past infection because IgG antibodies are detected as positive regardless of the time of infection or due to cross-reaction with other trematodes. Also, it can be false negative in cases with mild infection because of low levels of specific IgG antibody [4]. Bile examination is important for the diagnosis of C. sinensis infection. In the present case, adult worms were identified in the bile during the biliary drainage procedure. Additionally, polymerase chain reaction (PCR) assays as molecular diagnostic methods for clonorchiasis have been studied extensively in re cent years. Due to the similar morphologies of eggs of the Opisthorchiidae trematodes, this technology should be widely performed to assist in the definite diagnosis.
In addition, radiologic examinations, such as magnetic resonance cholangiopancreatography and CT scan, have made remarkable contributions in the diagnosis of clonorchiasis. Dilation, increased periductal echogenicity, and stricture of the intrahepatic bile ducts can be attributed to changes due to C. sinensis infection. Diffuse dilatation of the intrahepatic bile ducts up to the peripheral margin of the liver is observed; however, larger intrahepatic and extrahepatic bile ducts are usually not dilated or minimally dilated. These findings reflect the pathophysiology of bile ducts in C. sinensis. Adult C. sinensis worms usually reside in the medium-sized or small intrahepatic bile ducts. Typical findings of C. sinensis are peripheral intrahepatic dilation of biliary tree, and whitish floating materials in the gallbladder. This case revealed mild intrahepatic bile duct dilation and hyperechoic materials in the CBD and gallbladder. Prior to ERCP, she was diagnosed with stones in the CBD and acute calculus cholecystitis. CBD was blocked with C. sinensis worms in spite of mild infection.
According to World Health Organization recommendations, treatment with a dose of 25 mg/kg thrice daily for two consecutive days can achieve cure rates of 93.9–100% [15]. We treated patient with three times of praziquantel for one day, as the recommended standard regimen.
In conclusion, C. sinensis indwelling in the bile duct can persist for many years because of the lack of characteristic symptoms. The possibility of C. sinensis infection should be considered in patients with biliary symptoms. Even people living in non-endemic areas or with no history of raw freshwater fish intake, can be infected as a result of indirect transmission of C. sinensis through contaminated utensils.
Notes
Conflict of Interest
The authors have no conflicts to disclose.
