INTRODUCTION
Pancreatic cancer is the fourth-leading cause of cancer deaths in United States; it typically has an extremely poor prognosis, with a median five-year survival of around 2%. Because many patients diagnosed with pancreatic cancer have locally advanced or unresectable disease, the mainstay of treatment is chemotherapy rather than surgical treatment. Since Burris et al. [1] first reported gemcitabine-based chemotherapy for pancreatic cancer, a number of studies on gemcitabine in combination with various cytotoxic agents for advanced pancreatic cancer had been published [2]. Furthermore, preclinical studies have demonstrated the role of epidermal growth factor receptor (EGFR)-mediated signaling in the tumorigenesis of pancreatic cancer [3], and erlotinib, a small molecule tyrosine kinase inhibitor with specificity for EGFR, has been developed. Approved by the United States Food and Drug Administration in 2005, erlotinib in combination with gemcitabine has been used as the first-line therapy for treatment of patients with locally advanced and metastatic pancreatic cancer [4]. Because erlotinib acts relatively specifically on tumor cells, it is less toxic than conventional chemotherapy; the common side effects include acneiform (papulopustular) rash, diarrhea, edema, pruritus, dry skin, and alopecia [5]. Although there is a report of pneumatosis cystoides intestinalis (PCI) associated with the use of erlotinib in patients with lung cancer receiving erlotinib [6], it has not yet been reported in patients with pancreatic cancer patients receiving erlotinib. The authors experienced a case of PCI in a 66-year old woman during chemotherapy with a combination of gemcitabine and erlotinib for treatment of advanced pancreatic cancer accompanied by hepatic metastasis; we report our experience with this case and review the related literature.
CASE
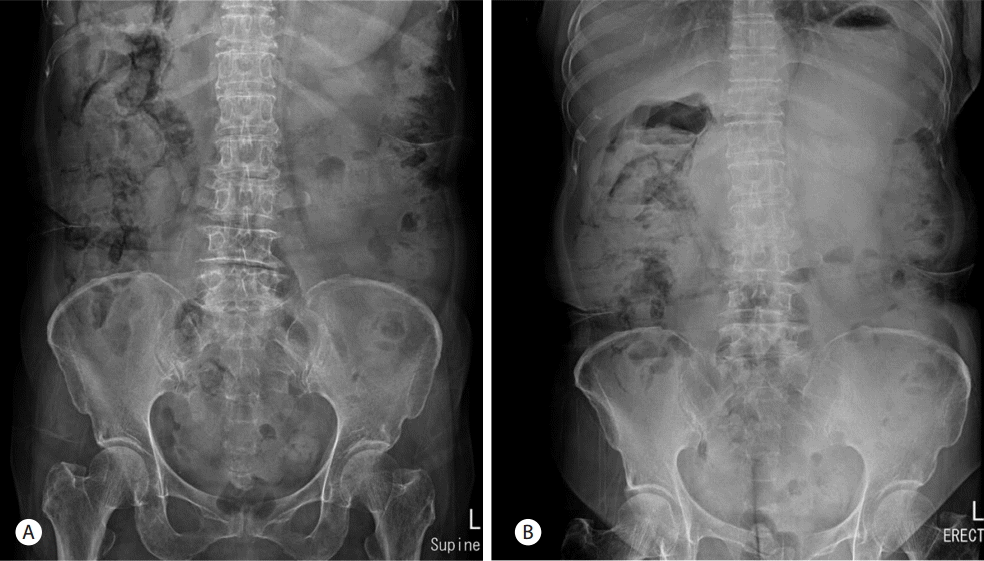
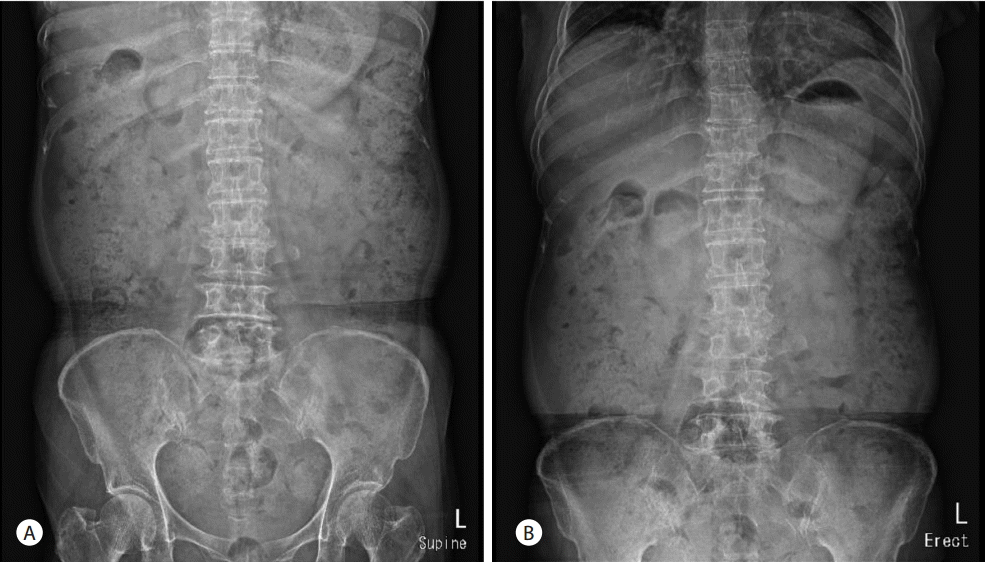
A 66-year-old woman was admitted to the hospital with a one month history of intermittent epigastric pain. She had no specific medical history except for taking oral medications for noninsulin-dependent diabetes mellitus about three months before admission. The patient had also no history of alcohol abuse or smoking. No abnormal findings were found in blood tests at admission, except for elevated carbohydrate antigen 19-9 (CA 19-9) levels of 8,967 U/mL. Abdominal computerized tomography (CT) scans revealed a 2.6 cm mass lesion around the pancreas neck (Fig. 1A, 1B) as well as multiple enlarged lymph nodes in peripancreatic areas including the para-aortic area. In addition, a metastatic lesion approximately 1 cm in size was observed in segment 4 of the liver (Fig. 1C). Accordingly, she was clinically diagnosed with advanced pancreatic cancer (T4 N1 M1, AJCC 7th edition). She underwent endoscopic ultrasound (EUS)-guided fine-needle aspiration (FNA) for histologic confirmation, but only necrotic tissues were obtained. It was still unclear whether repeat EUS-FNA would success and percutaneous approach to the hepatic lesion was not safe in interventional radiologist`s opinion. Thus laparoscopic excisional biopsy was conducted for the hepatic lesion, and histologic and immunohistochemical features showed positivity for CK7, CK19, and negative finding for CK 20 (Fig. 2). She was subsequently diagnosed with adenocarcinoma. She started chemotherapy with gemcitabine 1,000 mg/m2 weekly for three of every four weeks (last week rest) and erlotinib 100 mg orally once daily. She was maintained on this chemotherapy protocol without disease progression (according to revised RECIST criteria, version 1.1) for 13th cycles. Upon her admission to the hospital for the 14th cycles of chemotherapy, her blood pressure was 112/68 mmHg, pulse rate was 86 beats/minute, respiratory rate was 20 breaths/minute, and her body temperature was 36.5°C. She did not complain of abdominal pain. Physical examination revealed no tenderness or rebound tenderness. A blood test performed after admission found: white blood cell (WBC) count 8,460 /mm3 (72% neutrophils), hemoglobin 13.6 g/dL, aspartate aminotransferase 18 U/L, alanine aminotransferase 15 U/L, alkaline phosphatase 171 IU/L, r-glutamyltranspeptidase 48 IU/L, total bilirubin 0.9 mg/dL, amylase 33 U/L, and lipase 24 U/L. However, abdominal X-ray images showed an abnormal air shadow around the right-side colon, although pneumoperitoneum indicative of perforation was not observed (Fig. 3). Subsequent CT scans revealed intra-mural air along the ascending and the transverse colon, which was confirmed as valid findings for PCI (Fig. 4). The patient was observed while fasting for 3 days, but no specific symptoms were observed. A follow-up abdominal X-ray examination showed reduced abnormal air deposition, and oral intake was resumed (Fig. 5). We explained to the patient for possible complications and obtained consent for continued erlotinib-containing chemotherapy. Then, the patient was administered erlotinib-containing chemotherapy and was discharged from the hospital. A follow-up X-ray showed complete resolution (Fig. 5). Afterwards, the patient continued to receive chemotherapy with gemcitabine and erlotinib, and PCI was not observed through 16th cycles of chemotherapy. However, at this time, the patient was found to have disease progression, and is currently undergoing treatment with oral fluoropyrimidine (TS-1).
DISCUSSION
PCI is a relatively rare disease characterized by gas formation in the submucosa or subserosa of the gastrointestinal tract. The pathogenesis of PCI remains unclear, but its potential causes include the loss of mucosal integrity, elevated intraluminal pressure, and intraluminal gas production such as bacterial overgrowth. Its clinical manifestations vary from incidental finding such as observed in the current case, to life-threatening conditions such as bowel necrosis or perforation [7].
The potential causes of PCI can be divided into gastrointestinal causes such as peptic ulcer, inflammatory bowel disease, and Clostridium infection, and non-gastrointestinal causes such as chronic obstructive pulmonary disease and endoscopic procedure. In rare cases, chemotherapy may also cause PCI [8]. Furthermore, molecular targeted therapies recently used for treatment of a various diseases may cause PCI. Among these therapies, bevacizumab is most commonly associated with PCI [9]. Development of PCI has been reported during treatment with erlotinib, such as this case, but previous case reports occurred in patients with lung cancer; thus, to our knowledge, this is the first case of PCI in a patient with pancreatic cancer.
The pathophysiology of gastrointestinal toxicity caused by EGFR tyrosine kinase inhibitors such as erlotinib is not yet known, but there is a convincing hypothesis that EGF may be involved in the maintenance of mucosal integrity [10]. In other words, EGF deficiency within intestinal mucosa due to erlotinib treatment may interfere with mucosal integrity, causing diarrhea, nausea, and vomiting. In rare cases, PCI-associated moving of the bowel gas from the lumen to the bowel wall may develop [11].
PCI has also been associated with conventional chemotherapy such as cyclophosphamide, methotrexate, vincristine, doxorubicin, daunorubicin, cytarabine, fluorouracil, paclitaxel, docetaxel, etoposide, irinotecan, and cisplatin [12-14]. Thus, the development of PCI due to gemcitabine-based therapy cannot be completely excluded for the patient in this case. All the above-mentioned reports found neutropenia to be an important risk factor for PCI; however, the patient in this case did not show evidence of decreased neutrophil counts in a blood test performed at the time of PCI diagnosis. Therefore, PCI may be more likely to occur due to erlotinib than gemcitabine.
PCI or bowel perforation during targeted molecular therapy typically occurs within three months (range 1-13 months) after the start of treatment [9]. This timing may be explained by the fact that tumor response to targeted molecular therapy typically occurs after 1-2 months of treatment; thus, toxicity may also occur relatively early [9]. However, in this case, PCI occurred about 14 months after the start of erlotinib therapy. Therefore, clinicians should be aware that PCI might occur even after long-term erlotinib treatment.
Emergent surgery should be considered for patients presenting with acute abdominal symptoms on physical examination, including arterial pH <7.3, bicarbonate (HCO3) <20 mmol/L, elevated lactate level, elevated serum amylase level, or portal venous gas; otherwise, conservative treatment alone should be sufficient [8]. In addition, inhalation oxygen therapy may be helpful, but there are limitations to its use in clinical practice without standardized optimal amounts and durations [15]. Because the patient in the current case had no symptoms such as abdominal pain at diagnosis and no abnormal blood test findings, she underwent conservative treatment only and thereafter improved.
Even with successful treatment, PCI recurs in about 30-40% within 18 months [15]. However, in the current case, there has been no further recurrence, despite continued use of the causative drug erlotinib.
The authors experienced a case of PCI during gemcitabine and erlotinib combination therapy in a patient with pancreatic cancer and liver metastasis. Although the patient was immunocompromised while receiving chemotherapy, her lack of symptoms suggested a conservative treatment plan. Moreover, although she continued to use the causative drug erlotinib, PCI did not recur. However, because the pathogenesis of PCI associated with erlotinib is not yet fully understood, further studies are necessary to ensure patient safety, particularly for continued use of erlotinib.