INTRODUCTION
Pancreatic acinar cell carcinoma (ACC) is a very rare malignant neoplasm accounting for less than 1% of primary pancreatic neoplasms [1]. Because of the rarity of ACC, many issues such as diagnosis, surgical treatment, adjuvant therapy or therapeutic outcomes remain unclear. In general, although typical ductal adenocarcinoma is uncommonly confused for ACC, the preoperative diagnosis of ACC is difficult, imaging studies such as computed tomography (CT) or magnetic resonance imaging (MRI) only infrequently providing diagnostic clues and tissue diagnosis often requiring additional immunohistochemical assays [2,3]. ACC is often mistaken for pancreatic neuroendocrine tumor (PNET), solid pseudopapillary tumor (SPT) or pancreatoblastoma [2]. For treatment strategy, curative-intent surgery should be proposed when the disease in localized, not differing from that of pancreatic ductal adenocarcinoma (PDAC) [4]. However, the role of adjuvant chemotherapy has not been proven and the prognostic factors are rarely investigated. Here, we report a case of rapidly growing pancreatic ACC and review the literature to better understand this rare form of pancreatic malignancy.
CASE
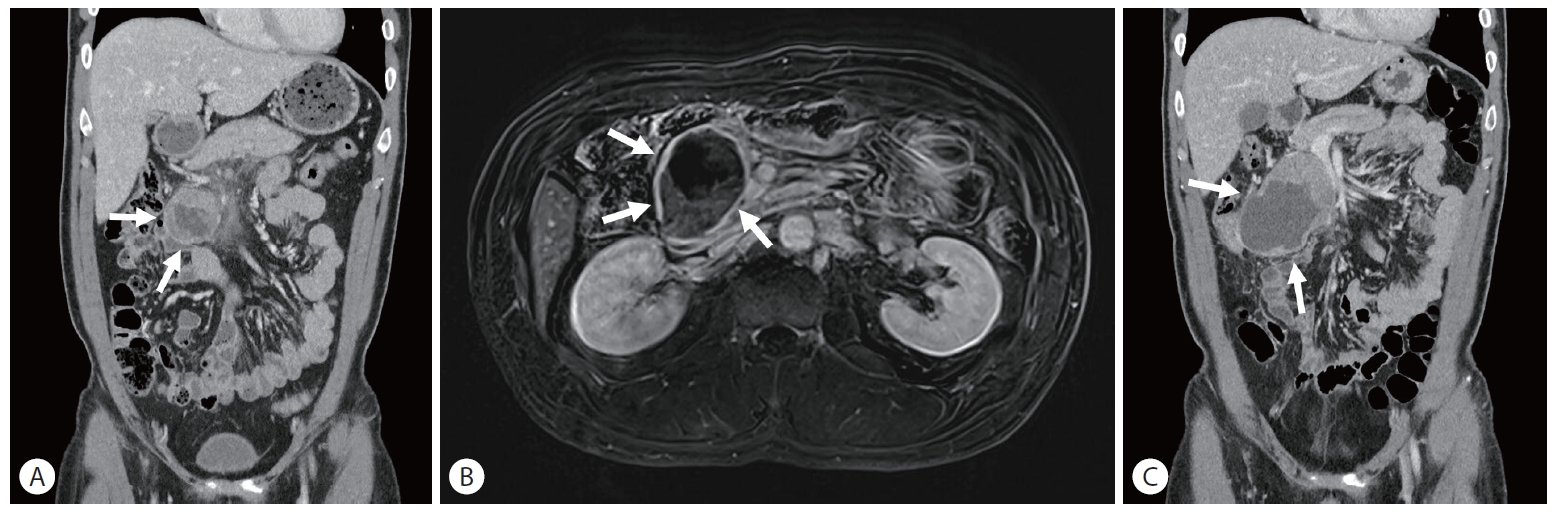
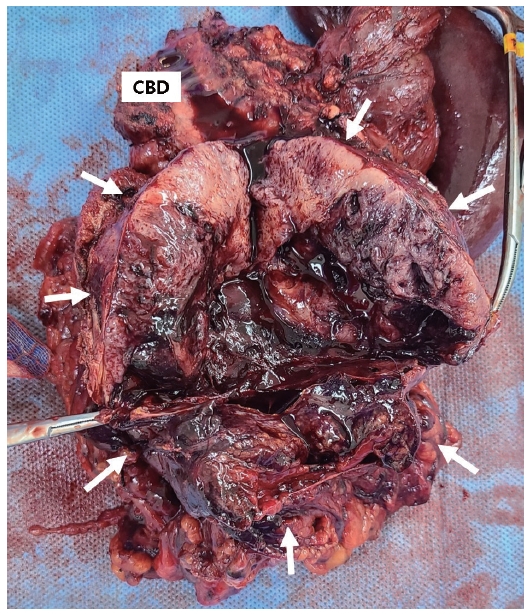
A 47-year-old male visited Emergency Department of Chung-Ang University Hospital due to severe pain in the epigastric area lasting 1 week. On physical examination, there was mild tenderness in the right upper quadrant of the abdomen, but the examination results were otherwise negative. His past medical history was unremarkable. CT of the abdomen and pelvis revealed a 4.7 cm heterogeneously enhanced solid and cystic mass located in the head of the pancreas without obstruction of the biliary or pancreatic duct (Fig. 1A). MRI showed a well-defined hypo-enhancing mass with peripheral diffusion restriction, internal necrosis and peripancreatic infiltration with minimal fluid collection (Fig. 1B). The patient’s serum lipase level was elevated at 2,138 IU/L (reference 67-21), while serum amylase level was 40 IU/L (reference 20-84) within normal range. All other laboratory parameters showed results within normal limits including carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9). CEA was 1.19 ng/mL (reference 0-3.8) and CA19-9 was 12.4 U/mL (reference 0-32). Owing to the patient’s private business schedule, he refused to undergo operation at the initial visit and returned 2 months later. Over those 2 months, the necrotic mass in the pancreas head had grown up to 11 cm, compressing the duodenum, superior mesenteric vein, and proximal transverse colon (Fig. 1C). However, because the tumor was thought to be resectable, without further work-up such as preoperative histologic diagnosis, surgery was performed. Exploration for the mass demonstrated involvement of the entire head of pancreas, transverse colon and its mesentery. Pylorus preserving pancreatoduodenectomy with segmental resection of transverse colon was performed (Fig. 2). Histopathological examination of the resected specimen revealed that the lesion consisted of highly cellular malignant epithelial tumor cells with scarce stroma and hemorrhage (Fig. 3A). Tumor cells were characterized by round nuclei with prominent nucleoli and acinar patterns (Fig. 3B). Immunohistochemistry demonstrated that the tumor cells were positive for trypsin (Fig. 3C), cytokeratin (CK), CK7, and E-cadherin. They were negative for CK19, chromogranin, synaptophysin and beta-catenin. These findings were consistent with those of pancreatic ACC. Tumor cells were observed to extend to the peripancreatic soft tissue without the presence of perineural invasion or regional lymph node metastasis. All surgical margins were tumor-free. The patient’s postoperative course was uneventful. He rejected adjuvant chemotherapy and was doing well without recurrence for 12 months.
DISCUSSION
Pancreatic ACC is a rare neoplasm, although acinar cells are predominant in the normal pancreas [1]. ACC have an expansile growth pattern with relatively good demarcation, so the invasion of the common bile duct is less than that of PDAC [2]. Therefore, there are more cases of non-specific symptoms such as abdominal pain than jaundice. Pancreatic ACC is often misdiagnosed as PDAC, PNET, or SPT. Approximately 50% of patients experience hyperlipasemia, and 10-15% of patients develop lipase hypersecretion syndrome, a type of paraneoplastic syndrome characterized by subcutaneous fat necrosis, polyarthralgia, and hypereosinophilia [3]. Pancreatic ACC is usually an exophytic, well-marginated, and hypovascular mass on CT and MRI. And it may contain cystic areas due to necrosis in case of large size [4]. Unlike ACC, PDAC is not well marginated and functioning PNET is more vascular. But, similar to ACC, non-functioning PNET may present as large well-marginated masses with internal hemorrhagic-cyst areas [4]. And typical CT appearance of SPT is a large well-encapsulated mass with solid and cystic components caused by hemorrhagic degeneration [5]. In terms of pathological diagnosis, ACC presents with different histological patterns, the neoplastic cells are uniform and arranged in architectural patterns, most typically acinar or solid [2]. The most common pancreatic neoplasm that are confused for ACC are PNET and SPT because of similar architectural pattern. Therefore, immunohistochemical staining for pancreatic enzymes including trypsin, chymotrypsin are used for diagnosis [3]. Trypsin is found to be the most commonly expressed enzyme followed by lipase, chymotrypsin and amylase. Compared to other markers, synaptophysin, chromogranin A, E-cadherin were markers for PNET and vimentin, nuclear labeling of β-catenin were markers for SPT [6]. Unlike that in PDAC, mutations of the K-ras oncogene, and the tumor suppressor gene p53 are not frequent in pancreatic ACC, however, chromosomal imbalances are common [7,8].
We searched the medical literature for studies describing therapeutic management and clinical outcomes for ACC. Case series and case reports with more than four patients were included. Among a total 142 manuscripts, 16 studies were included and Table 1 shows the summary of the included studies [1-3,9-21]. A total 1,901 patients were identified from the included studies had a mean age of 62 years, and 61% were men. In the present review, 40% of patients with ACC were located at pancreatic head and 41% of patients had resectable disease (stage I/II). There were three nation-wide study using national database, Japan pancreatic cancer registry, Surveillance, Epidemiology, and End Results (SEER) and National Cancer Database (NCDB) [13-15]. Among them, two studies from USA compared the clinical features and outcomes between ACC and PDAC [14,15]. Patients with ACC were more likely to be male (63.5% vs. 49.9%, 53.6% vs. 48.4%) and white (83.4% vs. 81.3%, 84.7% vs. 80.3%). ACC was more frequently located in the body/tail of the pancreas compared to PDAC (27.4% vs. 18.2%, 32.8% vs. 16.4%). More patients with ACC were presented with localized disease (34.6% vs. 22.4%, 14.7% vs. 8.4%) and curative resection rate was higher in the patients with ACC than in those with PDAC (64.35 vs. 55.9%, 69.1% vs. 27.3%). Overall 5-year survival rate for patients with ACC was significantly better than that of patients with PDAC (42.8% vs. 3.8%) [13]. In all three national wide studies, surgical resection significantly improved overall 5-year survival rate of the patients with ACC (43.9% vs. 0.0%, 72% vs. 22%, 36.2% vs. 10.4%). Surgery with any form of adjuvant therapy was associated with a better survival compared to patients who received surgery alone (41.2% vs. 32.7%) [15]. Since no standard chemotherapeutic regimens have yet been established for ACC, similar chemotherapeutic regimens to those for PDAC have often been used. Recently, it has been reported that the administration of platinum- or irinotecan- containing chemotherapy regimens such as FOLFIRINOX, are effective for advanced ACC and further more prospective clinical trials are needed [22,23]. Multivariate cox proportional hazard regression model suggested that survival of the patients with ACC was significantly better than that of the patients with PDAC (hazard ratio 0.24, 95% confidence interval 0.22-0.27) [14].
Pancreatic ACC is a rare malignant disease that cannot be easily differentiated from other common pancreatic tumors preoperatively. However, compared with PDAC, ACC seems to have a better prognosis, especially after resection. Therefore, for the relatively younger male patients with large pancreatic tumor with cystic component like the present case, surgeons should keep ACC in mind and should perform resection and adjuvant chemotherapy actively.