요약췌장의 점액성 낭성 종양(mucinous cystic neoplasm)은 국내 다기관 연구에 의하면 전체 췌장 낭성 종양의 약 25%를 차지하고 있다. 대부분 중년 여성에게 호발하고, 병변은 췌장 체부 및 미부에 위치하며, 점액성 낭선암(mucinous cystadenocarcinoma)으로 악화될 수 있는데, 크기가 서서히 증가하므로 이의 파열은 매우 드문 합병증이다. 저자들은 72세 남성에서 발생한 췌장 점액성 낭선암의 자발 파열 증례를 문헌 고찰과 함께 보고하는 바이다.
AbstractThe pancreatic cystic lesions are known to be incidentally found up to 10-15% of patients undergoing cross-sectional imaging. And the prevalence of mucinous cystic neoplasm which has malignant potential is known to be up to 25% of all pancreatic cystic neoplasm in South Korea. The symptoms included abdominal pain, palpable mass, weight loss, loss of appetite, jaundice, asymptomatic and etc. However, spontaneous rupture of pancreatic mucinous cystadenocarcinoma (MCAC) is an extremely rare complication. Here we report a case of spontaneous rupture of pancreatic MCAC in a 72-year-old male with review of the literature. To the best of our knowledge, this is the first ruptured case of pancreatic MCAC in male patient.
INTRODUCTIONThe pancreatic cystic lesions are known to be incidentally found up to 10-15% of patients undergoing cross-sectional imaging [1]. And, in Korea, the prevalence of mucinous cystic neoplasm (MCN) which has malignant potential is known to be up to 25% of all pancreatic cystic neoplasm [2]. The symptoms include abdominal pain, palpable mass, weight loss, loss of appetite, jaundice, asymptomatic and etc. However, spontaneous rupture of pancreatic mucinous cystadenocarcinoma (MCAC) is extremely rare complication. Here we report a case of spontaneous rupture of pancreatic MCAC in a 72-year-old male with review of the literature. To the best of our knowledge, this is the first ruptured case of pancreatic MCAC in male patient.
CASEA 72-year-old male visited outpatient clinic with a complaint of epigastric pain for 2 weeks. He was a social drinker and never-smoker. He denied any specific past medical history including pancreatitis as well as abdominal trauma. His brother was expired by stomach cancer about 5 years before.
When his first visit, blood pressure was 130/80 mmHg, pulse rate 70 bpm, respiration rate 20 bpm and body temperature 36.7°C. On physical examination, there was mild tenderness on epigastric area without rebound tenderness and no palpable abnormal mass. Laboratory data showed normal levels of complete blood cell count and blood chemistry test including serum amylase, lipase, and liver function test. There was mild elevation of alkaline phosphatase (334 IU/L). The tumor marker, CEA was within normal range (3.0 ng/mL), and CA19-9 was elevated (310 U/mL).
A plain abdomen x-ray did not show any abnormal finding. Computerized tomography (CT) scan showed about 15 cm sized cystic mass in the pancreatic body and tail which compressed the posterior wall of the stomach. In the lower part of main cystic mass, irregular cystic wall thickening and daughter cysts were observed, and a nodule around 1cm was showed in front of the cyst (Fig. 1). Magnetic resonance cholangiopancreatography showed normal common bile duct and pancreatic duct without any stricture or dilatation. There was no evidence of definite communication between the main pancreatic duct and cystic tumor (Fig. 2). Elective endoscopic ultrasonography and surgery was planned for the lesion with suspicious malignant change.
After 18th day of first visit, he went to the emergency room by severe abdominal pain on the left upper quadrant (LUQ) which was abrupt onset, non-relieving sharp nature. His blood pressure was 104/71 mmHg, heart rate 97 bpm, respiration rate 20 bpm, and body temperature 36.8°C. Physical examination showed severe LUQ tenderness without remarkable rebound tenderness. There were no abnormalities on the laboratory findings except mild leukocytosis (9,800/mm3). Emergent CT scan showed irregular high attenuation within the cystic lesion with wall defect around the spleen. And, fluid collection was noted in the bilateral paracolic gutter and pelvic cavity as well as in the periphery of the liver (Fig. 3).
Having the diagnosis of a ruptured pancreatic mucinous cystic tumor with hemoperitoneum, emergent surgery was performed. Open laparotomy revealed a large quantity of blood clots in the peritoneal cavity and multiple scattered nodules on the omentum, small bowel mesentery and the rectal shelf. The cystic lesions had infiltrated into the posterior wall and the serosa of the greater curvature of the stomach with disrupting areas, and there was a large quantity of hematoma within the cyst. Splenic artery, the feeding artery was ligated and hematoma were evacuated, then the ruptured arteries were treated with hemostatic procedure. The frozen biopsy of the nodules of peritoneum, mesentery of the small and large bowel were compatible with mucinous cystadenocarcinoma. Final pathologic finding was the cysts was consisted of the columnar epithelial cells, which infiltrated the normal tissues and contained a large amount of mucus in the cytoplasm (Fig. 4). He received chemotherapy with gemcitabine and erlotinib, however expired because of the aspiration pneumonia after 189 days of operation.
DISCUSSIONThe pancreatic cystic lesions are known to be incidentally found up to 10-15% of patients undergoing cross-sectional imaging [1]. According to a nationwide report of Korea, most common type of the cystic tumor of pancreas is intraductal papillary mucinous neoplasm (IPMN; 39.5%), and the second is MCN (25.2%). MCAC takes up to 29.9% of MCN by the pathologic diagnosis [2].
MCN mostly occurs in the body and tail, especially in middle-aged female in their 40’s and 50’s. However, little is known about the differences by sex in its clinical characteristics, because it is very rare in male. In one nationwide study of Japan [3], among 156 histopathologically confirmed MCN patients, male was only three (1.9%, 26 year old with minimally invasive cancer, and 36 & 72 year old with adenoma).
Most of the size of cystic tumors are large with a mean length of 10.5 cm and usually a single or multiple daughter cysts less than 2 cm size may appear [4,5]. The cystic mass is round and multi-lobulated, and generally have no communication with the pancreatic duct. The cystic wall is lined by the tall, columnar, mucin-secreting epithelium with frequent papillary proliferation, however, calcification is rare [6]. The characteristic pathologic finding of pancreatic MCN is ‘ovarian-type stroma’, which is subepithelial tissue composed of densely packed, spindle-shaped cells with round or elongated nuclei and sparse cytoplasm [3].
Pancreatic MCN has malignant potential as well as IPMN has. The prognosis is known to largely be favorable because it grows relatively slowly [7,8]. Hodgkinson et al. [9] reported a 68% of 5 year survival rate in cases of complete resection. Zamboni et al. [10] suggested that the most important prognostic factor is extent of invasion; and multilocularity, papillary projection, mural nodule or loss of ovarian-like stroma would have been a predictive factor for malignancy.
Spontaneous rupture of pancreatic MCAC is an extremely rare complication. To the best of our knowledge, only three cases have been reported. Smithers et al. [11] reported a case of spontaneous rupture of MCN in a 33-year-old pregnant woman at seven weeks of gestation in 1986. Murai et al. [12] reported a rupture of anaplastic MCAC in a 98-year-old female in 2006. And, Ozden et al. [13] reported a rupture of MCAC in a 32-year-old pregnant woman at 36 weeks of gestation in 2007. Our case is the first report of spontaneous ruptured pancreatic MCAC in a male patient, and we summarized the previous reported ruptured cases in Table 1.
As seen in the case by Smithers et al. [11] and Ozden et al. [13], pancreatic MCN that developed during pregnancy might rapidly growing, which could led to perforation and can cause fetal intrauterine growth retardation. Thus, the possible hormonal dependency of tumor was suggested. Actual progesterone receptors were demonstrated in papillary-cystic tumors of pancreas [14]. On the other hands, hormonal receptors had not been demonstrated in other studies, leaving the matter in debate [13].
Recently, various new combination chemotherapy regimen were established and applied to patients with advanced unresectable metastatic pancreatic cancer, such as erlotinib or nab-paclitaxel combination, or 5-fluouracil (FU) + irinotecan + oxaliplatin (FOLFIRI-NOX) combination. In our case, the patient received gemcitabine + erlotinib combination chemotherapy. Erlotinib is known as epidermal growth factor receptor targeting tyrosine kinase inhibitor, and the erlotinib + gemcitabine combination chemotherapy showed the better response than gemcitabine alone, on time to tumor progression and 1-year overall survival [15]. However, in case of malignant transformation of MCN, the better regimen is not established yet, so more study is warranted for clear this issue.
In conclusion, as seen by the cases by Murai et al. [12] and this case, MCN and MCAC had grown gradually by long period with having potential to rupture. Therefore, large pancreatic MCN in pregnant women and also in elderly patients should be considered early resection.
REFERENCES1. Teresa K, Carlos FC. Diagnosis and management of pancreatic cystic neoplasms. Hematol Oncol Clin North Am 2015;29:655-674.
2. Yoon WJ, Yoon YB, Lee KH, et al. The cystic neoplasms of the pancreas in Korea. Korean J Med 2006;70:261-267.
3. Yamao K, Yanagisawa A, Takahashi K, et al. Clinicopathological features and prognosis of mucinous cystic neoplasm with ovarian-type stroma. A multi-institutional study of the Japan pancreas society. Pancreas 2011;40:67-71.
5. Fugazzola C, Procacci C, Andreis IAB, et al. Cystic tumors of the pancreas; evaluation by ultrasonography and computed tomography. Gastrointest Radiol 1991;16:53-61.
6. Lee YC, Song SY, Chung JB, et al. Nine cases of mucinous cystic neoplasm of the pancreas. Korean J Gastroenterol 1994;26:728-736.
7. Chen J, Baithum SI. Morphological study of 391 cases of exocrine pancreatic tumors with special reference to the classification of exocrine pancreatic carcinoma. J Pathol 1985;146:17.
8. Compagno J, Oertel JE. Mucinous cystic neoplasms of the pancreas with overt and latent malignancy (cystadenocarcinoma and cystadenoma). a clinicopathologic study of 41 cases. Am J Clin Pathol 1978;69:573-580.
9. Hodgkinson DJ, ReMine WH, Weiland LH. Pancreatic cystadenoma; a clinicopathologic study of 45 cases. Arch Surg 1978;113:512.
10. Zamboni G, Scarpa A, Bogina G, et al. Mucinous cystic tumors of the pancreas: clinicopathological features, prognosis, and relationship to other mucinous cystic tumors. Am Surg Pathol 1999;23:410-422.
11. Smithers BM, Welch C, Goodall P. Cystadenocarcinoma of the pancreas presenting in pregnancy. Br J Surg 1986;73:591.
12. Murai H, Morimoto S, Ohashi I, Koaishi K. A ninety-eight-year-old woman with ruptured pancreatic anaplastic mucinous cystadenocarcinoma. Nippon Ronen Igakkai Zasshi 2006;43:246-251.
13. Ozden S, Haliloglu B, Ilter E, Akin FT, Kebudi A, Peker O. An extremely rare cause of acute abdomen in pregnancy: ruptured pancreatic mucinous cystadenocardinoma. Pancreas 2007;34:474-476.
Fig. 1.CT findings (A) initial Abdominal CT scan shows cystic mass of the pancreatic body and tail. Suspicious lymph node is observed in the anterior wall (B) Abdominal CT scan shows same mass with irregular wall thickening and septated small cysts. CT, computerized tomography. 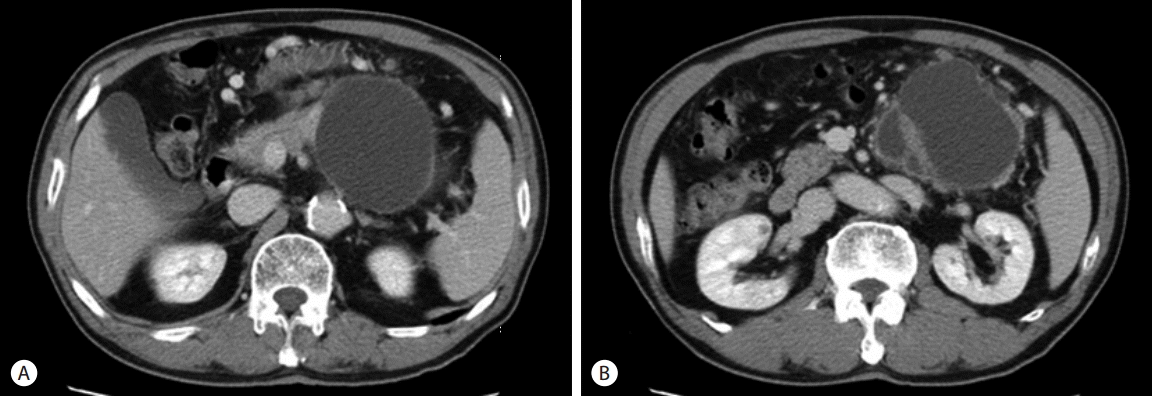
Fig. 2.MRCP finding. MRCP shows 15 cm size cystic mass with irregular wall and septated small cyst and normal pancreatic duct without definite communication with cystic mass. MRCP, magnetic resonance cholangiopancreatography. 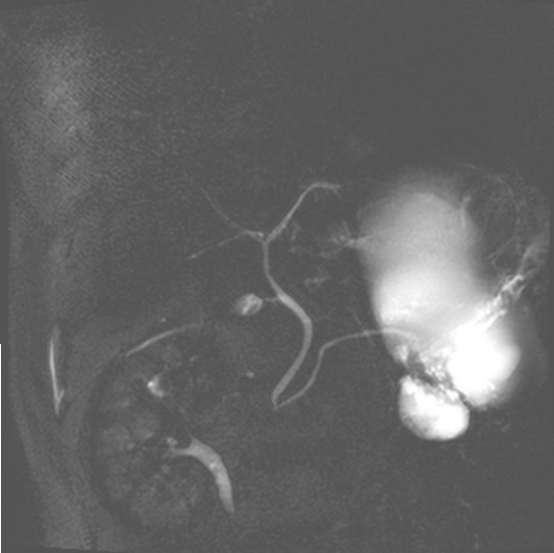
Fig. 3.CT findings (A) Unenhanced abdominal CT scan shows cystic mass with newly developed internal high attenuation and fluid collection in the right perihepatic space. (B) Enhanced abdominal CT scan shows partial loss of mass wall adjacent to spleen. CT, computerized tomography. 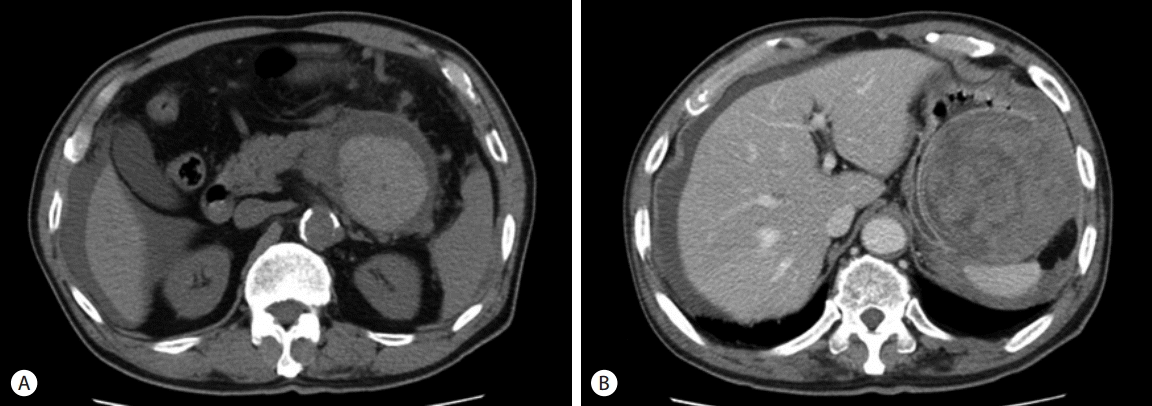
Fig. 4.Microscopic finding shows infiltrating adenocarcinoma with luminal and cytoplasmic mucin and irregular grandular shape (H&E stain, ×100). 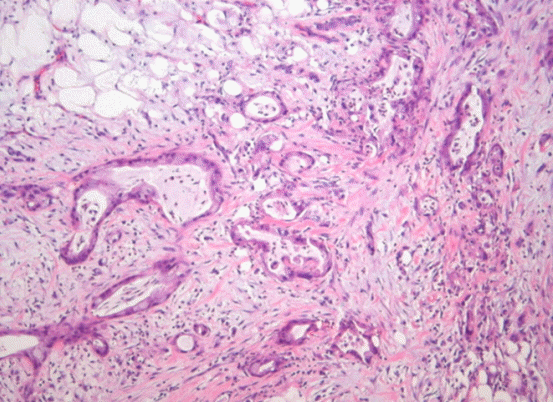
Table 1.Summary of ruptured case of pancreatic MCN in the literature
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||